Oxidopamine
| Oxidopamine | |
|---|---|
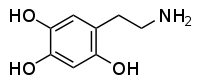 | |
| IUPAC name 5-(2-Aminoethyl)benzene-1,2,4-triol | |
| Identifiers | |
| CAS number | 1199-18-4 |
| PubChem | 4624 |
| ChemSpider | 4463 |
| UNII | 8HW4YBZ748 |
| KEGG | D05294 |
| ChEMBL | CHEMBL337702 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C8H11NO3 |
| Molar mass | 169.18 g/mol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Oxidopamine, also known as 6-hydroxydopamine (6-OHDA) or 2,4,5-trihydroxyphenethylamine, is a neurotoxic synthetic organic compound used by researchers to selectively destroy dopaminergic and noradrenergic neurons in the brain. 6-OHDA is thought to enter the neurons via the dopamine and noradrenaline (norepinephrine) reuptake transporters. Oxidopamine is often used in conjunction with a selective noradrenaline reuptake inhibitor (such as desipramine) to selectively destroy dopaminergic neurons only.
The main use for oxidopamine in scientific research is to induce Parkinsonism in laboratory animals such as mice, rats and monkeys, in order to develop and test new medicines and treatments for Parkinson's disease. In order to induce this condition in animals, around 70% of the dopaminergic neurons in the substantia nigra of the brain must be destroyed, and this is achieved either with oxidopamine or MPTP. Both these agents likely destroy neurons by generating reactive oxygen species such as superoxide radical. Oxidopamine toxicity in neonatal rodents is also used as an animal model for the Lesch-Nyhan syndrome.[1]
See also
References
- ↑ Breese GR, Knapp DJ, Criswell HE, Moy SS, Papadeas ST, Blake BL (2005). "The neonate-6-hydroxydopamine-lesioned rat: a model for clinical neuroscience and neurobiological principles". Brain Res. Brain Res. Rev. 48 (1): 57–73. doi:10.1016/j.brainresrev.2004.08.004. PMID 15708628.
| |||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||