5-HT2A receptor
The mammalian 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR).[1] This is the main excitatory receptor subtype among the GPCRs for serotonin (5-HT), although 5-HT2A may also have an inhibitory effect[2] on certain areas such as the visual cortex and the orbitofrontal cortex. This receptor was first given importance as the target of serotonergic psychedelic drugs such as LSD. Later it came back to prominence because it was also found to be mediating, at least partly, the action of many antipsychotic drugs, especially the atypical ones.
5-HT2A may be a necessary receptor for the spread of the human polyoma virus called JC virus.[3]
Downregulation of post-synaptic 5-HT2A receptor is an adaptive process provoked by chronic administration of SSRIs and classical antipsychotics. Deceased suicidal and otherwise depressed patients have had more 5-HT2A receptors than normal patients. These findings suggest that post-synaptic 5-HT2A overdensity is involved in the pathogenesis of depression.[4]
History
Serotonin receptors were split into two classes by Gaddum and Picarelli when it was discovered that some of the serotonin-induced changes in the gut could be blocked by morphine, whilst the remainder of the response was inhibited by dibenzyline leading to the naming of M and D receptors respectively. 5-HT2A is thought to correspond to what was originally described as D subtype of 5-HT receptors by Gaddum and Picarelli.[5] In the pre-molecular-cloning era when radioligand binding and displacement was the only major tool, spiperone and LSD were shown to label two different serotonin receptors, and neither of them displaced morphine, leading to naming of the 5-HT1, 5-HT2 and 5-HT3 receptors, corresponding to high affinity sites from LSD, spiperone and morphine respectively.[6] Later it was shown that the 5-HT2 was very close to 5-HT1C and thus were clubbed together, renaming the 5-HT2 into 5-HT2A. Thus the 5-HT2 receptor family is composed of three separate molecular entities: the 5-HT2A (formerly known as 5-HT2 or D), the 5-HT2B (formerly known as 5-HT2F) and the 5-HT2C (formerly known as 5-HT1C) receptors.[7]
Distribution
5-HT2A is expressed widely throughout the central nervous system (CNS). It is expressed near most of the serotoninergic terminal rich areas, including neocortex (mainly prefrontal, parietal, and somatosensory cortex) and the olfactory tubercle. Especially high concentrations of this receptor on the apical dendrites of pyramidal cells in layer V of the cortex may modulate cognitive processes,[8][9][10] by enhancing glutamate release followed by a complex range of interactions with the 5-HT1A,[11] GABAA,[12] adenosine A1,[13] AMPA,[14] mGluR2/3,[15] mGlu5,[16] and OX2 receptors.[17][18] In the rat cerebellum, the protein has also been found in the Golgi cells of the granular layer,[19] and in the Purkinje cells.[20][21]
In the periphery, it is highly expressed in platelets and many cell types of the cardiovascular system, in fibroblasts, and in neurons of the peripheral nervous system. Additionally, 5-HT2A mRNA expression has been observed in human monocytes.[22]
Signaling cascade
The 5-HT2A receptor is known primarily to couple to the Gαq signal transduction pathway. Upon receptor stimulation with agonist, Gαq and β-γ subunits dissociate to initiate downstream effector pathways. Gαq stimulates phospholipase C (PLC) activity, which subsequently promotes the release of diacylglycerol (DAG) and inositol triphosphate (IP3), which in turn stimulate protein kinase C (PKC) activity and Ca2+ release.[23]
There are many additional signal cascade components that include the formation of arachidonic acid through PLA2 activity, activation of phospholipase D, Rho/Rho kinase, and ERK pathway activation initiated by agonist stimulation of the receptor.[citation needed]
Effects
Physiological processes mediated by the receptor include:
- CNS: neuronal excitation, behavioural effects, learning, anxiety
- smooth muscle: contraction (in gastrointestinal tract & bronchi)
- vasoconstriction / vasodilation
- platelets: aggregation
- Activation of the 5-HT2A receptor with DOI produces potent anti-inflammatory effects in several tissues including cardiovascular and gut. Other 5-HT2A agonists like LSD also have potent anti-inflammatory effects against TNF-alpha-induced inflammation.[24][25]
- Activation of the 5-HT2A receptor in hypothalamus causes increases in hormonal levels of oxytocin, prolactin, ACTH, corticosterone, and renin.[26][27]
Ligands
Agonists
Activation of the 5-HT2A receptor is necessary for the effects of the "classic" psychedelics like LSD, psilocin and mescaline, which act as full or partial agonists at this receptor, and represent the three main classes of 5-HT2A agonists, the ergolines, tryptamines and phenethylamines, respectively. A very large family of derivatives from these three classes has been developed, and their structure-activity relationships have been extensively researched.[28][29] Agonists acting at 5-HT2A receptors located on the apical dendrites of pyramidal cells within regions of the prefrontal cortex are believed to mediate hallucinogenic activity. Newer findings reveal that psychoactive effects of classic psychedelics are mediated by the receptor heterodimer 5-HT2A–mGlu2 and not by monomeric 5-HT2A receptors.[30][31][32]
Full agonists
- 25I-NBOH and its 2-methoxy-analog 25I-NBOMe[33]
- 25C-NBOMe
- (R)-DOI
- TCB-2[34]
- Br-DFLY.[35]
- Mexamine is a full agonist to several serotonin receptors.
- O-4310, 5-HT2A selective, claimed to have 100x selectivity over 5-HT2C and be inactive at 5-HT2B
- PHA-57378, dual 5-HT2A / 5-HT2C agonist, anxiolytic effects in animal studies.[36]
Partial agonists
- Methysergide, a congener of methylergonovine, used in treatment of migraine blocks 5-HT2A and 5-HT2C receptors, but sometimes acts as partial agonist, in some preparations.
- The atypical antipsychotic aripiprazole is also a weak partial agonist.[37]
- OSU-6162 acts as a partial agonist at both 5-HT2A and dopamine D2 receptors
- NBOH-2C-CN, 100x selectivity for 5-HT2A over 5-HT2C, 46x selectivity over 5-HT2B.[38]
- (2S,6S)-2-(2,5-dimethoxy-4-bromobenzyl)-6-(2-methoxyphenyl)piperidine is a structurally constrained derivative of 25B-NBOMe, which acts as a potent partial agonist with 124x selectivity for 5-HT2A over 5-HT2C, making it the most selective agonist ligand identified to date.[39]
- Cannabidiol, a phytocannbinoid in Cannabis.[40]
- Efavirenz, an antiretroviral drug, produces psychiatric side effects thought to be mediated by 5-HT2A.[41]
Peripherally selective agonists
One effect of 5-HT2A receptor activation is a reduction in intraocular pressure, and so 5-HT2A agonists can be useful for the treatment of glaucoma. This has led to the development of compounds such as AL-34662 that are hoped to reduce pressure inside the eyes but without crossing the blood–brain barrier and producing hallucinogenic side effects.[42] Animal studies with this compound showed it to be free of hallucinogenic effects at doses up to 30 mg/kg, although several of its more lipophilic analogues did produce the head twitch response known to be characteristic of hallucinogenic effects in rodents.[43]
Silent antagonists
- Although ergot alkaloids are mostly nonspecific 5-HT receptor antagonists, a few ergot derivatives such as metergoline bind preferentially to members of the 5-HT2 receptor family.
- The discovery of Ketanserin was a landmark in the pharmacology of 5-HT2 receptors. Ketanserin, though capable of blocking 5-HT induced platelet adhesion, however does not mediate its well known antihypertensive action through 5-HT2 receptor family, but through its high affinity for alpha1 adrenergic receptors. It also has high affinity for H1 histaminergic receptors equal to that at 5-HT2A receptors. Compounds chemically related to ketanserin such as ritanserin are more selective 5-HT2A receptor antagonists with low affinity for alpha-adrenergic receptors. However, ritanserin, like most other 5-HT2A receptor antagonists, also potently inhibits 5-HT2C receptors.
- Nefazodone operates by blocking post-synaptic serotonin type-2A receptors and to a lesser extent by inhibiting pre-synaptic serotonin and norepinephrine (noradrenaline) reuptake.
- Atypical antipsychotic drugs like clozapine, olanzapine, quetiapine, risperidone and asenapine are relatively potent antagonists of 5-HT2A as are some of the lower potency old generation/typical antipsychotics. Other antagonists are MDL-100,907 (prototype of another new series of 5-HT2Aantagonists) and cyproheptadine.
- Pizotifen is a non-selective antagonist.[44]
- LY-367,265 - dual 5-HT2A antagonist / SSRI with antidepressant effects
- 2-alkyl-4-aryl-tetrahydro-pyrimido-azepines are subtype selective antagonists (35g: 60-fold).[45]
- AMDA and related derivatives are another family of selective 5-HT2A antagonists.[46][47][48][49][50]
- Hydroxyzine (Atarax)
- 5-MeO-NBpBrT
Inverse agonists
- AC-90179 - potent and selective inverse agonist at 5-HT2A, also 5-HT2C antagonist.[51][52]
- Nelotanserin (APD-125) - selective 5-HT2A inverse agonist developed by Arena Pharmaceuticals for the treatment of insomnia. APD-125 was shown to be effective and well tolerated in clinical trials.[53]
- Eplivanserin (Sanofi Aventis), a sleeping pill that reached phase II trials (but for which the application for approval was withdrawn), acts as a selective 5-HT2A inverse agonist.
- Pimavanserin (ACP-103) - more selective than AC-90179, orally active, antipsychotic in vivo, now in human clinical trials.[54][55][56][57]
- Volinanserin
Functional selectivity
5-HT2A-receptor ligands may differentially activate the transductional pathways (see above). Studies evaluated the activation of two effectors, PLC and PLA2, by means of their second messengers. Compounds displaying more pronounced functional selectivity are 2,5-DMA and 2C-N. The former induces IP accumulation without activating the PLA2 mediated response, while the latter elicits AA release without activating the PLC mediated response.[58]
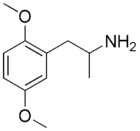

Recent research has suggested potential signaling differences within the somatosensory cortex between 5-HT2A agonists that produce headshakes in the mouse and those that do not, such as lisuride, as these agents are also non-hallucinogenic in humans despite being active 5-HT2A agonists.[59][60] One known example of differences in signal transduction is between the two 5-HT2A agonists serotonin and DOI that involves differential recruitment of intracellular proteins called β-arrestins, more specifically arrestin beta 2.[61][62]
Role of lipophilicity
A set of ligands were evaluated. For agonists, a highly significant linear correlation was observed between binding affinity and lipophilicity. For ligands exhibiting partial agonist or antagonist properties, the lipophilicity was consistently higher than would be expected for an agonist of comparable affinity.[63]
Genetics
The 5-HT2A receptors is coded by the HTR2A gene. In humans the gene is located on chromosome 13. The gene has previously been called just HTR2 until the description of two related genes HTR2B and HTR2C. Several interesting polymorphisms have been identified for HTR2A: A-1438G (rs6311), C102T (rs6313) and His452Tyr (rs6314). Many more polymorphisms exist for the gene. A 2006 paper listed 255.[64]
Associations with psychiatric disorders
Several studies have seen links between the -1438G/A polymorphism and mood disorders, such as bipolar disorder[65] and major depressive disorder.[66] A weak link with an odds ratio of 1.3 has been found between the T102C polymorphism and schizophrenia.[67] This polymorphism has also been studied in relation to suicide attempts, with a study finding excess of the C/C genotypes among the suicide attempters.[68] A number of other studies were devoted to finding an association of the gene with schizophrenia, with diverging results.[69]
These individual studies may, however, not give a full picture: A review from 2007 looking at the effect of different SNPs reported in separate studies stated that "genetic association studies [of HTR2A gene variants with psychiatric disorders] report conflicting and generally negative results" with no involvement, small or a not replicated role for the genetic variant of the gene.[70]
Treatment response
One study has found that genetic variations between individuals in the HTR2A gene may to some extent account for the difference in outcome of antidepressant treatment, so that patients suffering from major depressive disorder and treated with Citalopram may benefit more than others if they have one particular genotype.[71] In this study 768 single nucleotide polymorphism (SNP) across 68 genes were investigated and a SNP—termed rs7997012—in the second intron of the HTR2A gene showed significant association with treatment outcome.
Genetics seems also to be associated to some extent with the amount of adverse events in treatment of major depression disorder.[72][73]
One study has also linked abnormal 5-HT2A polymorphisms which may enhance receptor activity with Chronic Fatigue Syndrome.[74]
Neuroimaging
The 5-HT2A receptors may be imaged with PET-scanners using the fluorine-18-altanserin[75] and MDL 100,907[76] radioligands that binds to the neuroreceptor, e.g., one study reported a reduced binding of altanserin particularly in the hippocampus in patients with major depressive disorder.[77] Another PET study reported increased altanserin binding in the caudate nuclei in obsessive compulsive disorder patients compared to a healthy control group.[78]
Patients with Tourette's syndrome have also been scanned and the study found an increased binding of altanserin for patients compared to healthy controls.[79] The altanserin uptake decreases with age reflecting a loss of specific 5-HT2A receptors with age.[80][81][82] A study has also found a positive correlation among healthy subjects between altanserin binding and the personality trait neuroticism as measured by the NEO PI-R personality questionnaire.[83]
Role in virus endocytosis
5-HT2A may be a necessary receptor for clathrin mediated endocytosis of the human polyoma virus called JC virus, the causative agent of progressive multifocal leukoencephalopathy (PML), that enters cells such as oligodendrocytes, astrocytes, B lymphocytes, and kidney epithelial cells. These cells need to express both the alpha 2-6–linked sialic acid component of the 5-HT2A receptor in order to endocytose JCV.[3]
References
- ↑ Cook EH, Fletcher KE, Wainwright M, Marks N, Yan SY, Leventhal BL (August 1994). "Primary structure of the human platelet serotonin 5-HT2 receptor: identity with frontal cortex serotonin 5-HT2A receptor". J. Neurochem. 63 (2): 465–469. doi:10.1046/j.1471-4159.1994.63020465.x. PMID 8035173.
- ↑ Martin P, Waters N, Schmidt CJ, Carlsson A, Carlsson ML. (1998). "Rodent data and general hypothesis: antipsychotic action exerted through 5-Ht2A receptor antagonism is dependent on increased serotonergic tone". J Neural Transm 105 (4–5): 365–396. PMID 9720968.
- ↑ 3.0 3.1 Elphick GF, Querbes W, Jordan JA, Gee GV, Eash S, Manley K, Dugan A, Stanifer M, Bhatnagar A, Kroeze WK, Roth BL, Atwood WJ (2004). "The human polyomavirus, JCV, uses serotonin receptors to infect cells". Science 306 (5700): 1380–3. doi:10.1126/science.1103492. PMID 15550673.
- ↑ Eison AS, Mullins UL (1996). "Regulation of central 5-HT2A receptors: a review of in vivo studies". Behavioural Brain Research 73 (1–2): 177–81. doi:10.1016/0166-4328(96)00092-7. PMID 8788498.
- ↑ Sanders-Bush E, Mayer SE (2006). "Chapter 11: 5-Hydroxytryptamine (Serotonin): Receptor Agonists and Antagonists". In Brunton LL, Lazo JS, Parker K. Goodman & Gilman's the Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. ISBN 0-07-142280-3.
- ↑ George J. Siegel, R. Wayne Albers (2005). Basic neurochemistry: molecular, cellular, and medical aspects 1 (7th ed ed.). Academic Press. p. 241. ISBN 0-12-088397-X.
- ↑ Hoyer D, Hannon JP, Martin GR (April 2002). "Molecular, pharmacological and functional diversity of 5-HT receptors". Pharmacol. Biochem. Behav. 71 (4): 533–54. doi:10.1016/S0091-3057(01)00746-8. PMID 11888546.
- ↑ Aghajanian GK, Marek GJ (April 1999). "Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release". Brain Res. 825 (1-2): 161–71. doi:10.1016/S0006-8993(99)01224-X. PMID 10216183.
- ↑ Marek GJ, Wright RA, Gewirtz JC, Schoepp DD (2001). "A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex". Neuroscience 105 (2): 379–92. doi:10.1016/S0306-4522(01)00199-3. PMID 11672605.
- ↑ Bortolozzi A, Díaz-Mataix L, Scorza MC, Celada P, Artigas F (December 2005). "The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity". J. Neurochem. 95 (6): 1597–607. doi:10.1111/j.1471-4159.2005.03485.x. PMID 16277612.
- ↑ Amargós-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F (March 2004). "Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex". Cereb. Cortex 14 (3): 281–99. doi:10.1093/cercor/bhg128. PMID 14754868.
- ↑ Feng J, Cai X, Zhao J, Yan Z (September 2001). "Serotonin receptors modulate GABA(A) receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons". J. Neurosci. 21 (17): 6502–6511. PMID 11517239.
- ↑ Marek GJ (March 2009). "Activation of adenosine(1) (A(1)) receptors suppresses head shakes induced by a serotonergic hallucinogen in rats". Neuropharmacology 56 (8): 1082–7. doi:10.1016/j.neuropharm.2009.03.005. PMC 2706691. PMID 19324062.
- ↑ Zhang C, Marek GJ (January 2008). "AMPA receptor involvement in 5-hydroxytryptamine2A receptor-mediated pre-frontal cortical excitatory synaptic currents and DOI-induced head shakes". Progress in Neuro-psychopharmacology & Biological Psychiatry 32 (1): 62–71. doi:10.1016/j.pnpbp.2007.07.009. PMID 17728034.
- ↑ Gewirtz JC, Marek GJ (November 2000). "Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors". Neuropsychopharmacology 23 (5): 569–76. doi:10.1016/S0893-133X(00)00136-6. PMID 11027922.
- ↑ Marek GJ, Zhang C (September 2008). "Activation of metabotropic glutamate 5 (mGlu5) receptors induces spontaneous excitatory synaptic currents in layer V pyramidal cells of the rat prefrontal cortex". Neurosci. Lett. 442 (3): 239–43. doi:10.1016/j.neulet.2008.06.083. PMC 2677702. PMID 18621097.
- ↑ Lambe EK, Liu RJ, Aghajanian GK (November 2007). "Schizophrenia, hypocretin (orexin), and the thalamocortical activating system". Schizophr Bull 33 (6): 1284–90. doi:10.1093/schbul/sbm088. PMC 2779889. PMID 17656637.
- ↑ Liu RJ, Aghajanian GK (January 2008). "Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy". Proceedings of the National Academy of Sciences of the United States of America 105 (1): 359–64. doi:10.1073/pnas.0706679105. PMC 2224217. PMID 18172209.
- ↑ Geurts FJ, De Schutter E, Timmermans JP (June 2002). "Localization of 5-HT2A, 5-HT3, 5-HT5A and 5-HT7 receptor-like immunoreactivity in the rat cerebellum". Journal of chemical neuroanatomy 24 (1): 65–74. doi:10.1016/S0891-0618(02)00020-0. PMID 12084412.
- ↑ Maeshima T, Shutoh F, Hamada S, Senzaki K, Hamaguchi-Hamada K, Ito R, Okado N (August 1998). "Serotonin2A receptor-like immunoreactivity in rat cerebellar Purkinje cells". Neurosci. Lett. 252 (1): 72–74. doi:10.1016/S0304-3940(98)00546-1. PMID 9756362.
- ↑ Maeshima T, Shiga T, Ito R, Okado N (December 2004). "Expression of serotonin2A receptors in Purkinje cells of the developing rat cerebellum". Neurosci. Res. 50 (4): 411–417. doi:10.1016/j.neures.2004.08.010. PMID 15567478.
- ↑ Dürk T, Panther E, Müller T, Sorichter S, Ferrari D, Pizzirani C, Di Virgilio F, Myrtek D, Norgauer J, Idzko M. (May 2005). "5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes". Int Immunol 17 (5): 599–606. doi:10.1093/intimm/dxh242. PMID 15802305.
- ↑ Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB (2007). "Functional selectivity and classical concepts of quantitative pharmacology". J. Pharmacol. Exp. Ther. 320 (1): 1–13. doi:10.1124/jpet.106.104463. PMID 16803859.
- ↑ Yu B, Becnel J, Zerfaoui M, Rohatgi R, Boulares AH, Nichols CD (November 2008). "Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-_-induced inflammation with extraordinary potency". J. Pharmacol. Exp. Ther. 327 (2): 316–323. doi:10.1124/jpet.108.143461. PMID 18708586.
- ↑ Nau F, Yu B, Martin D, Nichols CD (2013). "Serotonin 5-HT2A Receptor Activation Blocks TNF-a Mediated Inflammation In Vivo". PLOS One 8 (10): e75426. doi:10.1371/journal.pone.0075426. PMID 24098382.
- ↑ Van De Kar, L. D.; Javed, A.; Zhang, Y.; Serres, F.; Raap, D. K.; Gray, T. S. (2001). "5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells". The Journal of neuroscience : the official journal of the Society for Neuroscience 21 (10): 3572–3579. PMID 11331386.
- ↑ Zhang Y, Damjanoska KJ, Carrasco GA, Dudas B, D'Souza DN, Tetzlaff J, Garcia F, Hanley NR, Scripathirathan K, Petersen BR, Gray TS, Battaglia G, Muma NA, Van de Kar LD (November 2002). "Evidence that 5-HT2A receptors in the hypothalamic paraventricular nucleus mediate neuroendocrine responses to (-)DOI". J Neurosci. 22 (21): 9635–9642. PMID 12417689.
- ↑ Nichols, DE (2004). "Hallucinogens.". Pharmacology & therapeutics 101 (2): 131–181. doi:10.1016/j.pharmthera.2003.11.002. PMID 14761703.
- ↑ Blaazer AR, Smid P, Kruse CG. Structure-activity relationships of phenylalkylamines as agonist ligands for 5-HT2A receptors. ChemMedChem. 2008 Sep;3(9):1299-309. PMID 18666267
- ↑ Moreno, J. L.; Muguruza, C.; Umali, A.; Mortillo, S.; Holloway, T.; Pilar-Cuéllar, F.; Mocci, G.; Seto, J.; Callado, L. F.; Neve, R. L.; Milligan, G.; Sealfon, S. C.; López-Giménez, J. F.; Meana, J. J.; Benson, D. L.; González-Maeso, J. (2012). "Identification of Three Residues Essential for 5-Hydroxytryptamine 2A-Metabotropic Glutamate 2 (5-HT2A{middle dot}mGlu2) Receptor Heteromerization and Its Psychoactive Behavioral Function". Journal of Biological Chemistry 287 (53): 44301–44319. doi:10.1074/jbc.M112.413161. PMC 3531745. PMID 23129762.
- ↑ González-Maeso, J.; Ang, R. L.; Yuen, T.; Chan, P.; Weisstaub, N. V.; López-Giménez, J. F.; Zhou, M.; Okawa, Y.; Callado, L. F.; Milligan, G.; Gingrich, J. A.; Filizola, M.; Meana, J. J.; Sealfon, S. C. (2008). "Identification of a serotonin/glutamate receptor complex implicated in psychosis". Nature 452 (7183): 93–97. doi:10.1038/nature06612. PMC 2743172. PMID 18297054.
- ↑ Moreno, J. L.; Holloway, T.; Albizu, L.; Sealfon, S. C.; González-Maeso, J. (2011). "Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists". Neuroscience Letters 493 (3): 76–79. doi:10.1016/j.neulet.2011.01.046. PMC 3064746. PMID 21276828.
- ↑ Braden MR, Parrish JC, Naylor JC, Nichols DE (2006). "Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists". Mol. Pharmacol. 70 (6): 1956–1964. doi:10.1124/mol.106.028720. PMID 17000863.
- ↑ McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, Nichols DE (September 2006). "1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists". Journal of Medical Chemistry 49 (19): 5794–803. doi:10.1021/jm060656o. PMID 16970404.
- ↑ Chambers JJ, Kurrasch-Orbaugh DM, Parker MA, Nichols DE (March 2001). "Enantiospecific synthesis and pharmacological evaluation of a series of super-potent, conformationally restricted 5-HT(2A/2C) receptor agonists". Journal of Medicinal Chemistry 44 (6): 1003–10. doi:10.1021/jm000491y. PMID 11300881.
- ↑ Ennis, M. D.; Hoffman, R. L.; Ghazal, N. B.; Olson, R. M.; Knauer, C. S.; Chio, C. L.; Hyslop, D. K.; Campbell, J. E.; Fitzgerald, L. W.; Nichols, N. F.; Svensson, K. A.; McCall, R. B.; Haber, C. L.; Kagey, M. L.; Dinh, D. M. (2003). "2,3,4,5-Tetrahydro- and 2,3,4,5,11,11a-hexahydro-1H-\1,4]diazepino\1,7-a]indoles: New templates for 5-HT2C agonists". Bioorganic & Medicinal Chemistry Letters 13 (14): 2369. doi:10.1016/S0960-894X(03)00403-7.
- ↑ Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu L, Sibley DR, Roth BL, Mailman R (May 2003). "Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology". Neuropsychopharmacology 28 (8): 1400–1411. doi:10.1038/sj.npp.1300203. PMID 12784105.
- ↑ Martin Hansen PhD. Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain. University of Copenhagen, 2011.
- ↑ Juncosa, J. I.; Hansen, M.; Bonner, L. A.; Cueva, J. P.; Maglathlin, R.; McCorvy, J. D.; Marona-Lewicka, D.; Lill, M. A.; Nichols, D. E. (2012). "Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands". ACS Chemical Neuroscience 4: 120717095020003. doi:10.1021/cn3000668.
- ↑ Russo, E. B.; Burnett, A.; Hall, B.; Parker, K. K. (2005). "Agonistic Properties of Cannabidiol at 5-HT1a Receptors". Neurochemical Research 30 (8): 1037–1043. doi:10.1007/s11064-005-6978-1. PMID 16258853.
- ↑ Gatch, M. B.; Kozlenkov, A.; Huang, R. Q.; Yang, W.; Nguyen, J. D.; González-Maeso, J.; Rice, K. C.; France, C. P.; Dillon, G. H.; Forster, M. J.; Schetz, J. A. (2013). "The HIV Antiretroviral Drug Efavirenz has LSD-Like Properties". Neuropsychopharmacology. doi:10.1038/npp.2013.135. PMID 23702798.
- ↑ Sharif NA, McLaughlin MA, Kelly CR (February 2007). "AL-34662: a potent, selective, and efficacious ocular hypotensive serotonin-2 receptor agonist". Journal of Ocular Pharmacology and Therapeutics 23 (1): 1–13. doi:10.1089/jop.2006.0093. PMID 17341144.
- ↑ May JA, Dantanarayana AP, Zinke PW, McLaughlin MA, Sharif NA (January 2006). "1-((S)-2-aminopropyl)-1H-indazol-6-ol: a potent peripherally acting 5-HT2 receptor agonist with ocular hypotensive activity". Journal of Medicinal Chemistry 49 (1): 318–328. doi:10.1021/jm050663x. PMID 16392816.
- ↑ Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4. Page 187
- ↑ Shireman BT, Dvorak CA, Rudolph DA, Bonaventure P, Nepomuceno D, Dvorak L, Miller KL, Lovenberg TW, Carruthers NI (March 2008). "2-Alkyl-4-aryl-pyrimidine fused heterocycles as selective 5-HT2A antagonists". Bioorganic & Medicinal Chemistry Letters 18 (6): 2103–8. doi:10.1016/j.bmcl.2008.01.090. PMID 18282705.
- ↑ Westkaemper RB, Runyon SP, Bondarev ML, Savage JE, Roth BL, Glennon RA (September 1999). "9-(Aminomethyl)-9,10-dihydroanthracene is a novel and unlikely 5-HT2A receptor antagonist". Eur. J. Pharmacol. 380 (1): R5–7. doi:10.1016/S0014-2999(99)00525-7. PMID 10513561.
- ↑ Westkaemper RB, Glennon RA (June 2002). "Application of ligand SAR, receptor modeling and receptor mutagenesis to the discovery and development of a new class of 5-HT2A ligands". Curr Top Med Chem 2 (6): 575–98. doi:10.2174/1568026023393741. PMID 12052195.
- ↑ Peddi S, Roth BL, Glennon RA, Westkaemper RB (December 2003). "Spiro[9,10-dihydroanthracene]-9,3'-pyrrolidine-a structurally unique tetracyclic 5-HT2A receptor antagonist". Eur. J. Pharmacol. 482 (1-3): 335–7. doi:10.1016/j.ejphar.2003.09.059. PMID 14660041.
- ↑ Runyon SP, Mosier PD, Roth BL, Glennon RA, Westkaemper RB (November 2008). "Potential modes of interaction of 9-aminomethyl-9,10-dihydroanthracene (AMDA) derivatives with the 5-HT2A receptor: a ligand structure-affinity relationship, receptor mutagenesis and receptor modeling investigation". J. Med. Chem. 51 (21): 6808–28. doi:10.1021/jm800771x. PMC 3088499. PMID 18847250.
- ↑ Wilson KJ, van Niel MB, Cooper L, Bloomfield D, O'Connor D, Fish LR, MacLeod AM (May 2007). "2,5-Disubstituted pyridines: the discovery of a novel series of 5-HT2A ligands". Bioorg. Med. Chem. Lett. 17 (9): 2643–8. doi:10.1016/j.bmcl.2007.01.098. PMID 17314044.
- ↑ Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, Harvey SC, Donohue E, Hansen HC, Andersson CM, Spalding TA, Gibson DF, Krebs-Thomson K, Powell SB, Geyer MA, Hacksell U, Brann MR (October 2001). "5-hydroxytryptamine2A receptor inverse agonists as antipsychotics". J. Pharmacol. Exp. Ther. 299 (1): 268–76. PMID 11561089.
- ↑ Vanover KE, Harvey SC, Son T, Bradley SR, Kold H, Makhay M, Veinbergs I, Spalding TA, Weiner DM, Andersson CM, Tolf BR, Brann MR, Hacksell U, Davis RE (September 2004). "Pharmacological characterization of AC-90179 [2-(4-methoxyphenyl)-N-(4-methyl-benzyl)-N-(1-methyl-piperidin-4-yl)-acetamide hydrochloride]: a selective serotonin 2A receptor inverse agonist". J. Pharmacol. Exp. Ther. 310 (3): 943–51. doi:10.1124/jpet.104.066688. PMID 15102927.
- ↑ Rosenberg, R; Seiden, DJ; Hull, SG; Erman, M; Schwartz, H; Anderson, C; Prosser, W; Shanahan, W; Sanchez, M; Chuang, E; Roth, T (2008). "APD125, a selective serotonin 5-HT2A receptor inverse agonist, significantly improves sleep maintenance in primary insomnia". Sleep 31 (12): 1663–71. PMC 2603489. PMID 19090322.
- ↑ Vanover KE, Weiner DM, Makhay M, Veinbergs I, Gardell LR, Lameh J, Del Tredici AL, Piu F, Schiffer HH, Ott TR, Burstein ES, Uldam AK, Thygesen MB, Schlienger N, Andersson CM, Son TY, Harvey SC, Powell SB, Geyer MA, Tolf BR, Brann MR, Davis RE (May 2006). "Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist". J. Pharmacol. Exp. Ther. 317 (2): 910–8. doi:10.1124/jpet.105.097006. PMID 16469866.
- ↑ Gardell LR, Vanover KE, Pounds L, Johnson RW, Barido R, Anderson GT, Veinbergs I, Dyssegaard A, Brunmark P, Tabatabaei A, Davis RE, Brann MR, Hacksell U, Bonhaus DW (August 2007). "ACP-103, a 5-hydroxytryptamine 2A receptor inverse agonist, improves the antipsychotic efficacy and side-effect profile of haloperidol and risperidone in experimental models". J. Pharmacol. Exp. Ther. 322 (2): 862–70. doi:10.1124/jpet.107.121715. PMID 17519387.
- ↑ Vanover KE, Betz AJ, Weber SM, Bibbiani F, Kielaite A, Weiner DM, Davis RE, Chase TN, Salamone JD (October 2008). "A 5-HT2A receptor inverse agonist, ACP-103, reduces tremor in a rat model and levodopa-induced dyskinesias in a monkey model". Pharmacol. Biochem. Behav. 90 (4): 540–4. doi:10.1016/j.pbb.2008.04.010. PMC 2806670. PMID 18534670.
- ↑ Abbas A, Roth BL (December 2008). "Pimavanserin tartrate: a 5-HT2A inverse agonist with potential for treating various neuropsychiatric disorders". Expert Opin Pharmacother 9 (18): 3251–9. doi:10.1517/14656560802532707. PMID 19040345.
- ↑ Moya PR, Berg KA, Gutiérrez-Hernandez MA, Sáez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP (2007). "Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine 5-HT2A and 5-HT2C receptors". J. Pharmacol. Exp. Ther. 321 (3): 1054–61. doi:10.1124/jpet.106.117507. PMID 17337633.
- ↑ González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007). "Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior". Neuron 53 (3): 439–52. doi:10.1016/j.neuron.2007.01.008. PMID 17270739.
- ↑ Cussac D, Boutet-Robinet E, Ailhaud MC, Newman-Tancredi A, Martel JC, Danty N, Rauly-Lestienne I (October 2008). "Agonist-directed trafficking of signalling at serotonin 5-HT2A, 5-HT2B and 5-HT2C-VSV receptors mediated Gq/11 activation and calcium mobilisation in CHO cells". Eur. J. Pharmacol. 594 (1-3): 32–8. doi:10.1016/j.ejphar.2008.07.040. PMID 18703043.
- ↑ Schmid CL, Raehal KM, Bohn LM (2008). "Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo". Proceedings of the National Academy of Sciences of the United States of America 105 (3): 1079–84. doi:10.1073/pnas.0708862105. PMC 2242710. PMID 18195357.
- ↑ Abbas A, Roth BL (2008). "Arresting serotonin". Proceedings of the National Academy of Sciences of the United States of America 105 (3): 831–2. doi:10.1073/pnas.0711335105. PMC 2242676. PMID 18195368.
- ↑ Parker MA, Kurrasch DM, Nichols DE (2008). "The role of lipophilicity in determining binding affinity and functional activity for 5-HT2A receptor ligands". Bioorg. Med. Chem. 16 (8): 4661–9. doi:10.1016/j.bmc.2008.02.033. PMC 2442558. PMID 18296055.
- ↑ "OSIRIS search results. Gene: HTR2A". Supplementary material to article
- Chambers JJ, Kurrasch-Orbaugh DM, Parker MA, Nichols DE (March 2001). "Enantiospecific synthesis and pharmacological evaluation of a series of super-potent, conformationally restricted 5-HT(2A/2C) receptor agonists". Journal of Medical Chemistry 44 (6): 1003–10. doi:10.1021/jm000491y. PMID 11300881.
- ↑ Chee IS, Lee SW, Kim JL, Wang SK, Shin YO, Shin SC, Lee YH, Hwang HM, Lim MR (2001). "5-HT2A receptor gene promoter polymorphism -1438A/G and bipolar disorder". Psychiatr. Genet. 11 (3): 111–114. doi:10.1097/00041444-200109000-00001. PMID 11702051.
- ↑ Choi MJ, Lee HJ, Lee HJ, Ham BJ, Cha JH, Ryu SH, Lee MS (2004). "Association between major depressive disorder and the -1438A/G polymorphism of the serotonin 2A receptor gene". Neuropsychobiology 49 (1): 38–41. doi:10.1159/000075337. PMID 14730199.
- ↑ Williams J, Spurlock G, McGuffin P, Mallet J, Nöthen MM, Gill M, Aschauer H, Nylander PO, Macciardi F, Owen MJ (1996). "Association between schizophrenia and T102C polymorphism of the 5-hydroxytryptamine type 2a-receptor gene. European Multicentre Association Study of Schizophrenia (EMASS) Group". The Lancet 347 (9011): 1294–1296. PMID 8622505.
- ↑ Vaquero-Lorenzo C, Baca-Garcia E, Diaz-Hernandez M, Perez-Rodriguez MM, Fernandez-Navarro P, Giner L, Carballo JJ, Saiz-Ruiz J, Fernandez-Piqueras J, Baldomero EB, de Leon J, Oquendo MA (July 2008). "Association study of two polymorphisms of the serotonin-2A receptor gene and suicide attempts". American Journal of Medical Genetics 147B (5): 645–9. doi:10.1002/ajmg.b.30642. PMID 18163387.
- ↑ Gene Overview of All Published Schizophrenia-Association Studies for HTR2A - SzGene database at Schizophrenia Research Forum.
- ↑ Serretti A, Drago A, De Ronchi D (2007). "HTR2A gene variants and psychiatric disorders: a review of current literature and selection of SNPs for future studies". Current medicinal chemistry 14 (19): 2053–69. doi:10.2174/092986707781368450. PMID 17691947.
- ↑ McMahon FJ, Buervenich S, Charney D, Lipsky R, Rush AJ, Wilson AF, Sorant AJ, Papanicolaou GJ, Laje G, Fava M, Trivedi MH, Wisniewski SR, Manji H (2006). "Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment". Am. J. Hum. Genet. 78 (5): 804–814. doi:10.1086/503820. PMC 1474035. PMID 16642436.
- ↑ Laje G, Paddock S, Manji H, Rush AJ, Wilson AF, Charney D, McMahon FJ (2007). "Genetic markers of suicidal ideation emerging during citalopram treatment of major depression". Am J Psychiatry 164 (10): 1530–1538. doi:10.1176/appi.ajp.2007.06122018. PMID 17898344.
- ↑ Laje G, McMahon FJ (2007). "The pharmacogenetics of major depression: past, present, and future". Biol. Psychiatry 62 (11): 1205–1207. doi:10.1016/j.biopsych.2007.09.016. PMID 17949692.
- ↑ Smith, A. K.; Dimulescu, I.; Falkenberg, V. R.; Narasimhan, S.; Heim, C.; Vernon, S. D.; Rajeevan, M. S. (2008). "Genetic evaluation of the serotonergic system in chronic fatigue syndrome". Psychoneuroendocrinology 33 (2): 188–197. doi:10.1016/j.psyneuen.2007.11.001. PMID 18079067.
- ↑ Lemaire C, Cantineau R, Guillaume M, Plenevaux A, Christiaens L (1 December 1991). "Fluorine-18-altanserin: a radioligand for the study of serotonin receptors with PET: radiolabeling and in vivo biologic behavior in rats". Journal of Nuclear Medicine 32 (12): 2266–2272. PMID 1744713.
- ↑ Lundkvist C, Halldin C, Ginovart N, Nyberg S, Swahn CG, Carr AA, Brunner F, Farde F (1996). "11C-MDL 100907, a radioligland for selective imaging of 5-HT2A receptors with positron emission tomography". Life Sci. 58 (10): PL 187–192. doi:10.1016/0024-3205(96)00013-6. PMID 8602111.
- ↑ Mintun MA, Sheline YI, Moerlein SM, Vlassenko AG, Huang Y, Snyder AZ (2004). "Decreased hippocampal 5-HT2A receptor binding in major depressive disorder: in vivo measurement with [18F]-altanserin positron emission tomography". Biological Psychiatry 55 (3): 217–24. doi:10.1016/j.biopsych.2003.08.015. PMID 14744461.
- ↑ Adams KH, Hansen ES, Pinborg LH, Hasselbalch SG, Svarer C, Holm S, Bolwig TG, Knudsen GM (2005). "Patients with obsessive-compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei". International Journal of Neuropsychopharmacology 8 (3): 391–401. doi:10.1017/S1461145705005055. PMID 15801987.
- ↑ Haugbøl S, Pinborg LH, Regeur L, Hansen ES, Bolwig TG, Nielsen FA, Svarer C, Skovgaard LT, Knudsen GM (2007). "Cerebral 5-HT2A receptor binding is increased in patients with Tourette's syndrome". Int. J. Neuropsychopharmacol. 10 (2): 245–52. doi:10.1017/S1461145706006559. PMID 16945163.
- ↑ Rosier A, Dupont P, Peuskens J, Bormans G, Vandenberghe R, Maes M, de Groot T, Schiepers C, Verbruggen A, Mortelmans L (1996). "Visualisation of loss of 5-HT2A receptors with age in healthy volunteers using [18F]-altanserin and positron emission tomographic imaging". Psychiatry Res. 68 (1): 11–22. doi:10.1016/S0925-4927(96)02806-5. PMID 9027929.
- ↑ Meltzer CC, Smith G, Price JC, Reynolds CF, Mathis CA, Greer P, Lopresti B, Mintun MA, Pollock BG, Ben-Eliezer D, Cantwell MN, Kaye W, DeKosky ST (1998). "Reduced binding of [18F]-altanserin to serotonin type 2A receptors in aging: persistence of effect after partial volume correction". Brain Res. 813 (1): 167–171. doi:10.1016/S0006-8993(98)00909-3. PMID 9824691.
- ↑ Adams KH, Pinborg LH, Svarer C, Hasselbalch SG, Holm S, Haugbøl S, Madsen K, Frøkjaer V, Martiny L, Olaf B. Paulson, Knudsen GM (2004). "A database of [18F]-altanserin binding to 5-HT2A receptors in normal volunteers: normative data and relationship to physiological and demographic variables". NeuroImage 21 (3): 1105–1113. doi:10.1016/j.neuroimage.2003.10.046. PMID 15006678.
- ↑ Frøkjær VG, Mortensen EL, Nielsen FÅ, Haugbøl S, Pinborg LH, Adams KH, Svarer C, Hasselbalch SG, Holm S, Paulson OB, Knudsen GM (2008). "Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder". Biological Psychiatry 63 (6): 569–76. doi:10.1016/j.biopsych.2007.07.009. PMID 17884017.
External links
- "5-HT2A". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology.
- 5-HT2A Receptor at the US National Library of Medicine Medical Subject Headings (MeSH)
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||



