5,7-Dihydroxytryptamine
| 5,7-Dihydroxytryptamine | |
|---|---|
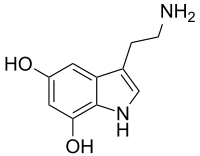 | |
| 3-(2-aminoethyl)-1H-indole-5,7-diol | |
| Other names 5,7-Dihydroxytryptamine | |
| Identifiers | |
| CAS number | 31363-74-3 |
| PubChem | 35781 |
| ChemSpider | 32913 |
| ChEMBL | CHEMBL26726 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C10H12N2O2 |
| Molar mass | 192.214 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
5,7-Dihydroxytryptamine (5,7-DHT) is a purported neurotoxin[1][2] used in scientific research to decrease concentrations of serotonin in the brain. The mechanism behind this effect isn't well-understood, but many believe (without clear substantiation) that it selectively kills serotonergic neurons, in a similar way that 6-hydroxydopamine (6-OHDA) is used to kill dopaminergic cells. What is known is that this compound is in fact not selective in depleting serotonin content, but also depletes norepinephrine. To selectively deplete serotonin stores, it is commonly administered in conjunction with desmethylimiprimine (desipramine), which inhibits the norepinephrine transporter.
See also
- 6-Hydroxydopamine (6-OHDA)
- para-Chlorophenylalanine (PCPA)
References
- ↑ Cairncross, KD; Cox, B; Forster, C; Wren, A (1977). "The ability of local injection of 6-OHDA, 5,6-DHT and 5,7-DHT into the olfactory bulbs to mimic the effects of bilateral bulbectomy in the rat proceedings". British Journal of Pharmacology 61 (1): 145P–146P. PMC 1667625. PMID 912193.
- ↑ Liu, J; Chu, YX; Zhang, QJ; Wang, S; Feng, J; Li, Q (2007). "5,7-dihydroxytryptamine lesion of the dorsal raphe nucleus alters neuronal activity of the subthalamic nucleus in normal and 6-hydroxydopamine-lesioned rats". Brain Research 1149: 216–22. doi:10.1016/j.brainres.2007.02.052. PMID 17376410.
| |||||||||||||||||||||||
| |||||