Substituted amphetamine
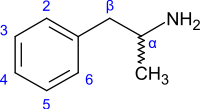
Substituted amphetamines are a chemical class of stimulants, entactogens, hallucinogens, and other drugs. They feature a phenethylamine core with a methyl group attached to the alpha carbon resulting in amphetamine, along with additional substitutions. Examples of amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, MDMA ("Ecstasy"), and DOM ("STP").
Amphetamine derivatives occur in nature, for example in the leaves of Ephedra and khat plants. These have been used since antiquity for their pharmacological effects. Amphetamines were first produced synthetically at the end of the 19th century. By the 1930s such synthetic amphetamines found use as decongestants in the symptomatic treatment of colds and also occasionally as psychoactive agents. Their effects on the central nervous system are diverse, but can be summarized by three overlapping types of activity: psychoanaleptic, hallucinogenic and empathogenic. Various amphetamines may cause these actions either separately or in combination.
List of substituted amphetamines
| Generic or Trivial Name | Chemical Name | # of Subs | Comments | Reference(s) |
|---|---|---|---|---|
| Amphetamine | α-Methyl-phenethylamine | 0 | ||
| Methamphetamine | N-Methylamphetamine, (1R,2S)- | 1 | ||
| Ethylamphetamine | N-Ethylamphetamine | 1 | ||
| Propylamphetamine | N-Propylamphetamine | 1 | ||
| Isopropylamphetamine | N-iso-Propylamphetamine | 1 | ||
| Phentermine | α-Methylamphetamine | 1 | ||
| Phenylpropanolamine (PPA) | β-Hydroxyamphetamine, (1R,2S)- | 1 | ||
| Cathine | β-Hydroxyamphetamine, (1S,2S)- | 1 | ||
| Cathinone | β-Ketoamphetamine | 1 | ||
| Ortetamine | 2-Methylamphetamine | 1 | ||
| 2-Fluoroamphetamine (2-FA) | 2-Fluoroamphetamine | 1 | ||
| 3-Methylamphetamine (3-MA) | 3-Methylamphetamine | 1 | ||
| 3-Fluoroamphetamine (3-FA) | 3-Fluoroamphetamine | 1 | ||
| Norfenfluramine | 3-Trifluoromethylamphetamine | 1 | ||
| 4-Methylamphetamine (4-MA) | 4-Methylamphetamine | 1 | ||
| para-Methoxyamphetamine (PMA) | 4-Methoxyamphetamine | 1 | ||
| para-Ethoxyamphetamine | 4-Ethoxyamphetamine | 1 | ||
| 4-Methylthioamphetamine (4-MTA) | 4-Methylthioamphetamine | 1 | ||
| Norpholedrine (α-Me-TRA) | 4-Hydroxyamphetamine | 1 | ||
| para-Bromoamphetamine (PBA, 4-BA) | 4-Bromoamphetamine | 1 | ||
| para-Chloroamphetamine (PCA, 4-CA) | 4-Chloroamphetamine | 1 | ||
| para-Fluoroamphetamine (PFA, 4-FA, 4-FMP) | 4-Fluoroamphetamine | 1 | ||
| para-Iodoamphetamine (PIA, 4-IA) | 4-Iodoamphetamine | 1 | ||
| Clobenzorex | N-(2-chlorobenzyl)-1-phenylpropan-2-amine | 1 | ||
| Dimethylamphetamine | N,N-Dimethylamphetamine | 2 | ||
| Benzphetamine | N-Benzyl-N-methylamphetamine | 2 | ||
| Selegiline | N-Methyl-N-propargylamphetamine, (R)- | 2 | ||
| Mephentermine | N-Methyl-α-methylamphetamine | 2 | ||
| Phenpentermine | α,β-Dimethylamphetamine | 2 | ||
| Ephedrine (EPH) | β-Hydroxy-N-methylamphetamine, (1R,2S)- | 2 | ||
| Pseudoephedrine (PSE) | β-Hydroxy-N-methylamphetamine, (1S,2S)- | 2 | ||
| Methcathinone | β-Keto-N-methylamphetamine | 2 | ||
| Ethcathinone | β-Keto-N-ethylamphetamine | 2 | ||
| Clortermine | 2-Chloro-α-methylamphetamine | 2 | ||
| Methoxymethylamphetamine (MMA) | 3-Methoxy-4-methylamphetamine | 2 | ||
| Fenfluramine | 3-Trifluoromethyl-N-ethylamphetamine | 2 | ||
| Dexfenfluramine | 3-Trifluoromethyl-N-ethylamphetamine, (S)- | 2 | ||
| 4-Methylmethamphetamine (4-MMA) | 4-Methyl-N-methylamphetamine | 2 | ||
| Para-methoxymethamphetamine (PMMA) | 4-Methoxy-N-methylamphetamine | 2 | ||
| para-Methoxyethylamphetamine (PMEA) | 4-Methoxy-N-ethylamphetamine | 2 | ||
| Pholedrine | 4-Hydroxy-N-methylamphetamine | 2 | ||
| Chlorphentermine | 4-Chloro-α-methylamphetamine | 2 | ||
| para-Fluoromethamphetamine (PFMA, 4-FMA) | 4-Fluoro-N-methylamphetamine | 2 | ||
| Xylopropamine | 3,4-Dimethylamphetamine | 2 | ||
| α-Methyldopamine (α-Me-DA) | 3,4-Dihydroxyamphetamine | 2 | ||
| Methylenedioxyamphetamine (MDA) | 3,4-Methylenedioxyamphetamine | 2 | ||
| Dimethoxyamphetamine (DMA) | X,X-Dimethoxyamphetamine | 2 | ||
| Nordefrin (α-Me-NE) | β,3,4-Trihydroxyamphetamine, (R)- | 3 | ||
| Oxilofrine | β,4-Dihydroxy-N-methylamphetamine | 3 | ||
| Aleph | 2,5-dimethoxy-4-methylthioamphetamine | 3 | ||
| Dimethoxybromoamphetamine (DOB) | 2,5-Dimethoxy-4-bromoamphetamine | 3 | ||
| Dimethoxychloroamphetamine (DOC) | 2,5-Dimethoxy-4-chloroamphetamine | 3 | ||
| Dimethoxyfluoroethylamphetamine (DOEF) | 2,5-Dimethoxy-4-fluoroethylamphetamine | 3 | ||
| Dimethoxyethylamphetamine (DOET) | 2,5-Dimethoxy-4-ethylamphetamine | 3 | ||
| Dimethoxyfluoroamphetamine (DOF) | 2,5-Dimethoxy-4-fluoroamphetamine | 3 | ||
| Dimethoxyiodoamphetamine (DOI) | 2,5-Dimethoxy-4-iodoamphetamine | 3 | ||
| Dimethoxymethylamphetamine (DOM) | 2,5-Dimethoxy-4-methylamphetamine | 3 | ||
| Dimethoxynitroamphetamine (DON) | 2,5-Dimethoxy-4-nitroamphetamine | 3 | ||
| Dimethoxypropylamphetamine (DOPR) | 2,5-Dimethoxy-4-propylamphetamine | 3 | ||
| Dimethoxytrifluoromethylamphetamine (DOTFM) | 2,5-Dimethoxy-4-trifluoromethylamphetamine | 3 | ||
| Methylenedioxymethamphetamine (MDMA) | 3,4-Methylenedioxy-N-methylamphetamine | 3 | ||
| Methylenedioxyethylamphetamine (MDEA) | 3,4-Methylenedioxy-N-ethylamphetamine | 3 | ||
| Methylenedioxyhydroxyamphetamine (MDOH) | 3,4-Methylenedioxy-N-hydroxyamphetamine | 3 | ||
| 2-Methyl-MDA | 3,4-Methylenedioxy-2-methylamphetamine | 3 | ||
| 5-Methyl-MDA | 4,5-Methylenedioxy-3-methylamphetamine | 3 | ||
| Methoxymethylenedioxyamphetamine (MMDA) | 3-Methoxy-4,5-methylenedioxyamphetamine | 3 | ||
| Trimethoxyamphetamine (TMA) | X,X,X-Trimethoxyamphetamine | 3 | ||
| Dimethylcathinone | β-Keto-N,N-dimethylamphetamine | 3 | ||
| Diethylcathinone | β-Keto-N,N-diethylamphetamine | 3 | ||
| Bupropion | β-Keto-3-chloro-N-tert-butylamphetamine | 3 | ||
| Mephedrone (4-MMC) | β-Keto-4-methyl-N-methylamphetamine | 3 | ||
| Methedrone (PMMC) | β-Keto-4-methoxy-N-methylamphetamine | 3 | ||
| Brephedrone (4-BMC) | β-Keto-4-bromo-N-methylamphetamine | 3 | ||
| Flephedrone (4-FMC) | β-Keto-4-fluoro-N-methylamphetamine | 3 | ||
History
Although the basic compound of the class, amphetamine, was synthesized earlier, Ephedra was used 5000 years ago in China as a medicinal plant; its active ingredients are alkaloids ephedrine, pseudoephedrine, norephedrine (phenylpropanolamine) and norpseudoephedrine (cathine). Natives of Yemen and Ethiopia have a long tradition of chewing khat leaves to achieve a stimulating effect. The active substances of khat are cathinone and to a lesser extent cathine.[1]
Amphetamine was first synthesized in 1887 by Romanian chemist Lazăr Edeleanu and did not attract special attention.[2] MDMA was produced in 1912 (according to other sources in 1914[3]) as an intermediate product. However, this synthesis also went largely unnoticed.[4] In the 1920s, both methamphetamine and an optical isomer of amphetamine dextroamphetamine (D-amphetamines) were synthesized. This synthesis was a by-product of a search for ephedrine, a bronchodilator used to treat asthma extracted exclusively from natural sources. Over-the-counter use of amphetamines was initiated in early 1930s by the pharmaceutical company Smith, Kline & French (now part of GlaxoSmithKline), as a medicine (Benzedrine) for colds and nasal congestion. Subsequently, amphetamine was used in the treatment of narcolepsy, obesity, hay fever, orthostatic hypotension, epilepsy, Parkinson's disease, alcoholism and migraine.[2][5] The "reinforcing" effects of amphetamines were quickly discovered, and the misuse of amphetamines had been noted as far back as 1936.[5]
During World War II, amphetamines were used by the German military to keep their tank crews awake for long periods, and treat depression. It was noticed that extended rest was required after such artificially induced activity.[2]
The widespread use of amphetamines began in postwar Japan and quickly spread to other countries. Modified ("designer") amphetamines gained popularity since the 1960s, such as MDA and PMA.[5] MDMA was rediscovered and popularized by the American chemist Alexander Shulgin in 1965, after which it was used for some time in psychotherapy sessions.[4] In 1970, the United States adopted "the Controlled Substances Act" that limited non-medical use of amphetamines.[5] Street use of PMA was noted in 1972,[6] and in 1985, MDMA was banned by the US authorities in an emergency scheduling initiated by the US Drug Enforcement Agency.[7]
Since the mid-1990s, MDMA ("ecstasy") has become a popular entactogenic drug among the youth and quite often non-MDMA substances were sold as "ecstasy".[8] In the first legally sanctioned trials in the USA in over twenty years, the safety profile of MDMA has been demonstrated, and it has been shown to be a successful adjunct to psychotherapy in the management of treatment-resistant post-traumatic stress disorder (PTSD) in victims of sexual abuse and sufferers of other conditions.[9]
Structure
Amphetamines are a subgroup of the substituted phenethylamine class of compounds. Substitution of hydrogen atoms results in a large class of compounds. Typical reaction is substitution by methyl and sometimes ethyl groups at the amine and phenyl sites:[10][11][12]
| Substance | Substituents | Structure | ||||||
|---|---|---|---|---|---|---|---|---|
| N | α | β | phenyl group | |||||
| 2 | 3 | 4 | 5 | |||||
| Amphetamine (α-methylphenylethylamine) | -CH3 | | ||||||
| Methamphetamine (N-methylamphetamine) | -CH3 | -CH3 | | |||||
| Ephedrine pseudoephedrine | -CH3 | -CH3 | -OH | | ||||
| Cathinone | -CH3 | =O | | |||||
| Methcathinone (ephedrone) | -CH3 | -CH3 | =O | | ||||
| MDA (3,4-methylenedioxyamphetamine) | -CH3 | -O-CH2-O- | | |||||
| MDMA (3,4-methylenedioxymethamphetamine) | -CH3 | -CH3 | -O-CH2-O- | | ||||
| MDEA (3,4-methylenedioxy-N-ethylamphetamine) | -CH2-CH3 | -CH3 | -O-CH2-O- | | ||||
| EDMA (3,4-ethylenedioxy-N-methylamphetamine) | -CH3 | -CH3 | -O-CH2-CH2-O- | | ||||
| MBDB (N-methyl-1-(3,4-methylenedioxyphenyl)-2-aminobutane) | -CH3 | -CH2-CH3 | -O-CH2-O- | | ||||
| PMA (para-methoxyamphetamine) | -CH3 | -O-CH3 | | |||||
| PMMA (para-methoxymetamphetamine) | -CH3 | -CH3 | -O-CH3 | | ||||
| 4-MTA (4-methylthioamphetamine) | -CH3 | -S-CH3 | | |||||
| 3,4-DMA (3,4-dimethoxyamphetamine) | -CH3 | -O-CH3 | -O-CH3 | | ||||
| 3,4,5-TMA (3,4,5-trimethoxyamphetamine, α-methylmescaline) | -CH3 | -O-CH3 | -O-CH3 | -O-CH3 | | |||
| DOM (2,5-dimethoxy-4-methylamphetamine) | -CH3 | -O-CH3 | -CH3 | -O-CH3 | 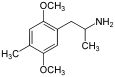 | |||
| DOB (2,5-dimethoxy-4-bromoamphetamine) | -CH3 | -O-CH3 | -Br | -O-CH3 | 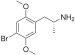 | |||
Legal status
| Agents | Legal status by 2009.[13][14][15] | ||
|---|---|---|---|
| UN Convention on Psychotropic Substances of 1971[16] | US | Russia | |
| Amphetamine (racemic) | Schedule II | Schedule II | Schedule II |
| Dextroamphetamine (D-amphetamine) | Schedule II | Schedule II | Schedule I |
| Levamphetamine (L-amphetamine) | Schedule II | Schedule II | Schedule III |
| Methamphetamine | Schedule II | Schedule II | Schedule I |
| Cathinone Methcathinone | Schedule I | Schedule I | Schedule I |
| MDA, MDMA, MDEA | Schedule I | Schedule I | Schedule I |
| PMA | Schedule I | Schedule I | Schedule I |
| DOB, DOM, 3,4,5-TMA | Schedule I | Schedule I | Schedule I |
See also
- Substituted Phenethylamines
- Substituted Methylenedioxyphenethylamines
- Substituted cathinones
- 2Cs
- DOx
References
- ↑ Paul M Dewick (2002). Medicinal Natural Products. A Biosynthetic Approach. Second Edition. Wiley. pp. 383–384. ISBN 0-471-49640-5.
- ↑ 2.0 2.1 2.2 Snow, p. 1
- ↑ A. Richard Green et al. (2003). "The Pharmacology and Clinical Pharmacology of 3,4-Methylenedioxymethamphetamine (MDMA, "Ecstasy")". Pharmacological Reviews 55 (3): 463–508. doi:10.1124/pr.55.3.3. PMID 12869661.
- ↑ 4.0 4.1 Goldfrank, p. 1125
- ↑ 5.0 5.1 5.2 5.3 Goldfrank, p. 1119
- ↑ Liang Han Ling et al. (2001). "Poisoning with the recreational drug paramethoxyamphetamine ("death" )". The Medical Journal of Australia 174 (9): 453–5. PMID 11386590.
- ↑ Snow, p. 71
- ↑ Goldfrank, p. 1121
- ↑ Mithoefer M. et al. (2011). "The safety and efficacy of ±3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study". Journal of Psychopharmacology 25 (4): 439–52. doi:10.1177/0269881110378371. PMC 3122379. PMID 20643699.
- ↑ Gold frank, pp. 1125–1127
- ↑ Glennon, pp. 184–187
- ↑ Schatzberg, p.843
- ↑ "List of psychotropic substances under international control". International Narcotics Control Board. August 2003. May 2010 Edition
- ↑ "DEA Drug Scheduling". U.S. Drug Enforcement Administration. Retrieved 2009-11-17.
- ↑ "Resolution of RF Government of 30 June 1998 N 681 "On approval of list of drugs psychotropic substances and their precursors subject to control in the Russian Federation"". garant.ru (in Russian). Retrieved 2009-11-15.
- ↑ "Convention on Psychotropic Substances, 1971". United Nations.
Bibliography
- Ghodse, Hamid (2002). Drugs and Addictive Behaviour. A Guide to Treatment. 3rd Edition. Cambridge University Press. p. 501. ISBN 0-511-05844-6.
- Glennon, Richard A. (2008). The American Psychiatric Publishing textbook of substance abuse treatment. American Psychiatric Publishing. ISBN 978-1-58562-276-4. Unknown parameter
|part=ignored (help) - Goldfrank, Lewis R. and Flomenbaum, Neal (2006). Goldfrank's Toxicologic Emergencies, 8th Edition. McGraw Hill. ISBN 0-07-147914-7.
- Katzung, Bertram G. (2009). Basic & clinical pharmacology. 11th edition. McGraw-Hill Medical. ISBN 0-07-160405-7.
- Ledgard, Jared (2007). A Laboratory History of Narcotics. Volume 1. Amphetamines and Derivatives. Jared Ledgard. p. 268. ISBN 0-615-15694-0.
- Schatzberg, Alan F. and Nemeroff, Charles B. (2009). The American Psychiatric Publishing Textbook of Psychopharmacology. The American Psychiatric Publishing. ISBN 978-1-58562-309-9.
- Snow, Otto (2002). Amphetamine syntheses. Thoth Press. ISBN 0-9663128-3-X.
- UNODC (2009). World Drug Report 2009. United Nations. p. 306. ISBN 978-92-1-148240-9.
- Veselovskaya NV, Kovalenko AE (2000). Drugs. Properties, effects, pharmacokinetics, metabolism. MA: Triada-X. ISBN 978-5-94497-029-9.
| ||||||||||||||||||||||||||||||
| ||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||||||||||