4-Nitroaniline
| p-Nitroaniline | |
|---|---|
 | |
| Other names 4-nitroaniline | |
| Identifiers | |
| CAS number | 100-01-6 |
| ChemSpider | 13846959 |
| UNII | 1MRQ0QZG7G |
| ChEMBL | CHEMBL14282 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C6H6N2O2 |
| Molar mass | 138.12 g/mol |
| Appearance | yellow or brown powder |
| Odor | faint, ammonia-like |
| Density | 1.437 g/ml, solid |
| Melting point | 146-149 °C(lit.) |
| Boiling point | 332 °C |
| Solubility in water | 0.8 mg/ml at 18.5°C (IPCS) |
| Hazards | |
| MSDS | JT Baker |
| EU classification | |
| R-phrases | R23/24/25 R33 R52/53 |
| S-phrases | S28 S36/37 S45 S61 |
| Main hazards | Toxic |
| NFPA 704 |
 1
2
0
|
| Flash point | 199 °C; 390 °F; 472 K |
| Related compounds | |
| Related compounds | 2-Nitroaniline, 3-Nitroaniline |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
4-Nitroaniline, p-nitroaniline or 1-amino-4-nitrobenzene is an organic compound with the formula C6H6N2O2. It is an organic chemical compound, consisting of a phenyl group attached to an amino group which is para to a nitro group. This chemical is commonly used as an intermediate in the synthesis of dyes, antioxidants, pharmaceuticals and gasoline, in gum inhibitors, poultry medicines, and as a corrosion inhibitor.
Synthesis
It is produced industrially via the amination of 4-nitrochlorobenzene:[1]
- ClC6H4NO2 + 2 NH3 → H2NC6H4NO2 + NH4Cl
Below is a laboratory synthesis of p-nitroaniline from aniline. The key step in this reaction sequence is an electrophilic aromatic substitution to install the nitro group para to the amino group. After this reaction, a separation must be performed to remove 2-nitroaniline, which is also formed in a small amount during the reaction.[2]

Applications
4-Nitroaniline is mainly consumed industrially as a precursor to p-phenylenediamine, an important dye. The reduction is effected using iron metal and by catalytic hydrogenation.[1]
It is a starting material for the synthesis of Para Red, the first Azo dye:[3]
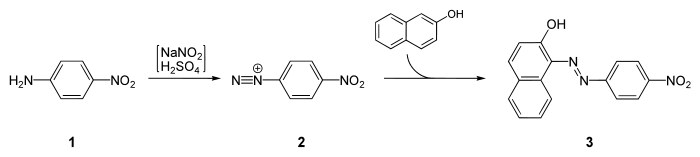
Toxicity
The compound is toxic by way of inhalation, ingestion, and absorption, and should be handled with care. Its LD50 in rats is 750 mg/kg when administered orally. p-Nitroaniline is particularly harmful to all aquatic organisms, and can cause long-term damage to the environment if released as a pollutant.
References
- ↑ 1.0 1.1 Gerald Booth "Nitro Compounds, Aromatic in Ullmann's Encyclopedia of Industrial Chemistry, 7th Ed.; Wiley-VCH: Weinheim, 2005. doi:10.1002/14356007.a17_411
- ↑ Mohrig, J.R.; Morrill, T.C.; Hammond, C.N.; Neckers, D.C. "Synthesis 5: Synthesis of the Dye Para Red from Aniline." Experimental Organic Chemistry. Freeman: New York, NY, 1997; pp 456-467.
- ↑ Williamson, Kenneth L. (2002). Macroscale and Microscale Organic Experiments, Fourth Edition. Houghton-Mifflin. ISBN 0-618-19702-8.
External links
- Safety (MSDS)data for p-nitroaniline
- MSDS Sheet for p-nitroaniline
- Sigma-Aldrich Catalog data
- CDC - NIOSH Pocket Guide to Chemical Hazards