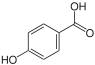
4-Hydroxybenzoic acid
| 4-Hydroxybenzoic acid | |
|---|---|
 |
 |
| 4-Hydroxybenzoic acid | |
| Other names p-Hydroxybenzoic acid | |
| Identifiers | |
| CAS number | 99-96-7 |
| PubChem | 135 |
| ChemSpider | 132 |
| EC number | 202-804-9 |
| DrugBank | DB04242 |
| KEGG | C00156 |
| ChEBI | CHEBI:30763 |
| ChEMBL | CHEMBL441343 |
| Jmol-3D images | Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | C7H6O3 |
| Molar mass | 138.121 g/mol |
| Appearance | white crystalline |
| Odor | odorless |
| Density | 1.46 g/cm³ |
| Melting point | 214.5 °C; 418.1 °F; 487.6 K |
| Solubility in water | 0.5 g/100 mL |
| Solubility | soluble in alcohol, ether, acetone slightly soluble in chloroform negligible in CS2 |
| log P | 1.58 |
| Acidity (pKa) | 4.54 |
| Hazards | |
| MSDS | HMDB |
| Main hazards | Irritant |
| NFPA 704 |
 0
2
0
|
| Autoignition temperature | 250 °C; 482 °F; 523 K |
| LD50 | 2200 mg/kg (oral, mouse) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
4-Hydroxybenzoic acid is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar organic solvents such as alcohols and acetone. 4-Hydroxybenzoic acid is primarily known as the basis for the preparation of its esters, known as parabens, which are used as preservatives in cosmetics and some ophthalmic solutions. It is isomeric with 2-hydroxybenzoic acid, known as salicylic acid, a precursor to aspirin.
Natural occurrences
Cryptanaerobacter phenolicus is a bacterium species that produces benzoate from phenol via 4-hydroxybenzoate.[1]
p-Hydroxybenzoic acid glucoside can be found in mycorrhizal and non-mycorrhizal roots of Norway spruces (Picea abies).[2]
Violdelphin is ananthocyanin, a type of plant pigments, found in blue flowers and incorporating two p-hydroxy benzoic acid residues, one rutinoside and two glucosides associated with a delphinidin.
Occurrences in food
4-Hydroxybenzoic acid can be found naturally in Cocos nucifera.[3] It is one of the main catechins metabolites found in humans after consumption of green tea infusions.[4] It is also found in wine[5] and in vanilla.
Açaí oil, obtained from the fruit of the açaí palm (Euterpe oleracea), is rich in p-hydroxybenzoic acid (892 ± 52 mg/kg).[6]
Chemical production
4-Hydroxybenzoic acid is produced commercially from potassium phenoxide and carbon dioxide in the Kolbe-Schmitt reaction.[7] It can also be produced in the laboratory by heating potassium salicylate with potassium carbonate to 240 °C, followed by treating with acid.[8]
Chemical reactions
4-Hydroxybenzoic acid has about one tenth the acidity of benzoic acid, having an acid dissociation constant Ka = 3.3 x 10−5 M at 19 °C.[citation needed] Its acid dissociation follows this equation:
- HOC6H4CO2H
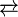 HOC6H4CO2− + H+
HOC6H4CO2− + H+
Safety
4-Hydroxybenzoic acid is a popular antioxidant in part because of its low toxicity. The LD50 is 2200 mg/kg in mice (oral).[citation needed]
References
- ↑ Cryptanaerobacter phenolicus gen. nov., sp. nov., an anaerobe that transforms phenol into benzoate via 4-hydroxybenzoate. Pierre Juteau, Valérie Côté, Marie-France Duckett, Réjean Beaudet, François Lépine, Richard Villemur and Jean-Guy Bisaillon, IJSEM, January 2005, vol. 55, no. 1, pages 245-250, doi:10.1099/ijs.0.02914-0
- ↑ Phenolics of mycorrhizas and non-mycorrhizal roots of Norway spruce. Babette Münzenberger, Jürgen Heilemann, Dieter Strack, Ingrid Kottke and Franz Oberwinkler, Planta, Volume 182, Number 1, pages 142-148, doi:10.1007/BF00239996
- ↑ Profiling C6–C3 and C6–C1 phenolic metabolites in Cocos nucifera. Gargi Dey, Moumita Chakraborty and Adinpunya Mitra, Journal of Plant Physiology, Volume 162, Issue 4, 22 April 2005, Pages 375-381 doi:10.1016/j.jplph.2004.08.006
- ↑ Catechin metabolites after intake of green tea infusions. P. G. Pietta, P. Simonetti, C. Gardana, A. Brusamolino, P. Morazzoni and E. Bombardelli, BioFactors, 1998, Volume 8, Issue 1-2, pp. 111–118, doi:10.1002/biof.5520080119
- ↑ Comparison of Phenolic Acids and Flavan-3-ols During Wine Fermentation of Grapes with Different Harvest Times. Rong-Rong Tian, Qiu-Hong Pan, Ji-Cheng Zhan, Jing-Ming Li, Si-Bao Wan, Qing-Hua Zhang and Wei-Dong Huang, Molecules, 2009, 14, pages 827-838, doi:10.3390/molecules14020827
- ↑ Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST (Jun 2008). "Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.)". J Agric Food Chem 56 (12): 4631–6. doi:10.1021/jf800161u. PMID 18522407.
- ↑ Edwin Ritzer and Rudolf Sundermann “Hydroxycarboxylic Acids, Aromatic” in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a13_519
- ↑ C. A. Buehler and W. E. Cate (1943), "p-Hydroxybenzoic acid", Org. Synth.; Coll. Vol. 2: 341
| ||||||||||||||||