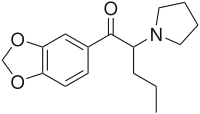
Methylenedioxypyrovalerone
 | |
|---|---|
 | |
| Systematic (IUPAC) name | |
| (RS)-1-(Benzo[d][1,3]dioxol-5-yl)-2-(pyrrolidin-1-yl)pentan-1-one | |
| Clinical data | |
| Legal status | Prohibited (S9) (AU) Schedule I (US) Illegal in Canada, Czech Republic, Denmark, Sweden and UK |
| Routes | Oral, Insufflation, Intravenous, Rectal, Vaporization |
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Primarily Urine (Renal) |
| Identifiers | |
| CAS number | 687603-66-3 24622-62-6 (HCl) |
| ATC code | ? |
| PubChem | CID 20111961 |
| ChemSpider | 16788110 |
| Chemical data | |
| Formula | C16H21NO3 |
| Mol. mass | 275.343 g/mol (freebase) |
| SMILES
| |
| |
| Physical data | |
| Melt. point | 209.3 °C (409 °F) |
| Boiling point | 476 °C (889 °F) |
| | |
Methylenedioxypyrovalerone (MDPV) is a psychoactive drug with stimulant properties which acts as a norepinephrine-dopamine reuptake inhibitor (NDRI).[1] It was first developed in the 1960s by a team at Boehringer Ingelheim.[2] MDPV remained an obscure stimulant until around 2004 when it was reportedly sold as a designer drug. Products labeled as bath salts containing MDPV were previously sold as recreational drugs in gas stations and convenience stores in the United States, similar to the marketing for Spice and K2 as incense.[3][4]
Incidents of psychological and physical harm have been attributed to MDPV use.[5][6]
Appearance
The hydrochloride salt exists as a very fine, hygroscopic, crystalline powder that tends to clump to itself, resembling something like powdered sugar. Its color can range from pure white to a yellowish-tan and has a slight odor that strengthens as it colors. Impurities are likely to consist of either pyrrolidine or alpha-dibrominated alkylphenones from either excess pyrrolidine or incomplete amination, respectively, during synthesis. These impurities likely account for its discoloration and fishy (pyrrolidine) or bromine-like odor, which worsens upon exposure to air, moisture, or bases.[7]
Pharmacology
Methylenedioxypyrovalerone has no record of FDA approved medical use.[8] Reportedly, it has four times the potency of methylphenidate (Ritalin, Concerta),[9] although its pharmacology has only recently been studied in detail.[10] MDPV has been shown to produce robust reinforcing effects and compulsive self-administration in rats, though this had already been provisionally established by a number of documented cases of misuse and addiction in humans, before the animal tests had been carried out.[11]
MDPV is the 3,4-methylenedioxy ring-substituted analog of the compound pyrovalerone, developed in the 1960s, which has been used for the treatment of chronic fatigue and as an anorectic, but caused problems of abuse and dependence. However, despite its structural similarity, the effects of MDPV bear little resemblance to other methylenedioxy phenylalkylamine derivatives such as 3,4-methylenedioxy-N-methylamphetamine (MDMA), instead producing primarily stimulant effects with only mild entactogenic qualities.
Other drugs with a similar chemical structure include α-pyrrolidinopropiophenone (α-PPP), 4'-methyl-α-pyrrolidinopropiophenone (M-α-PPP), 3',4'-methylenedioxy-α-pyrrolidinopropiophenone (MDPPP) and 1-phenyl-2-(1-pyrrolidinyl)-1-pentanone (α-PVP).
Effects
MDPV acts as a stimulant and has been reported to produce effects similar to those of cocaine, methylphenidate, and the amphetamines. The acute effects may include:[12]
Desired psychological effects
- euphoria
- increased alertness and awareness
- increased wakefulness and arousal
- increased energy and motivation
- mental stimulation/increased concentration
- increased sociability
- sexual stimulation/aphrodisiac effects[13]
- mild empathogenic effects
- diminished perception of the requirement for food and sleep
Description of effects
The primary psychological effects have a duration of roughly 3 to 4 hours, with after effects such as tachycardia, hypertension, and mild stimulation lasting from 6 to 8 hours.[12] High doses have been observed to cause intense, prolonged panic attacks in stimulant-intolerant users,[12] and there are anecdotal reports of psychosis from sleep withdrawal and addiction at higher doses or more frequent dosing intervals.[12] MDPV has been distinguished by some for its powers as an aphrodisiac. It has also been repeatedly noted for inducing strong cravings to re-administer.[12][14] Users have reported a compulsive desire to continuously re-dose, even following onset of the unpleasant side effects induced by prolonged use and higher doses.[citation needed]
Reported modalities of intake include oral consumption, insufflation, smoking, rectal and intravenous use. It is supposedly active at 3–5 mg, with typical doses ranging between 5–20 mg.[12]
Chemistry
MDPV can be prepared by modifying the alkylation-oxidation-bromination-amination route to cathinone analogs. This involves a Grignard alkylation of piperonal, oxidation of the resulting secondary alcohol back into a ketone, alpha halogenation of the aromatic ketone with bromine, and subsequent amination with pyrrolidine.[15]
The α,α-dibrominated or α-mono-brominated intermediate from the 3rd step bromination, and left behind by an expedited or incomplete final workup is the most likely contaminant/impurity to be seen in the final product using this method.[7] Bromination is likely to in excess, since excess pyrollidine amine will form a black precipitate that is difficult to separate.
Such brominated halo are of particular concern to eukaryote macro-biological organisms, and special consideration must be made when preparing MDPV for purposes of long-term biological testing on lab animals, especially in mammals, and especially where the possibility exists for diversion and subsequent ingestion by humans for recreational purposes. Preparation for biological purposes and testing must ensure a complete and thorough workup, including several washes with saturated sodium bicarbonate[7] solution after washing with water to remove the brominated intermediates before final salting of the freebase for recrystallization. Aside from the bromine ion being highly electronegative and reactive, the alkyl-bromine compounds often being alkylating agents, and brominated aromatic derivatives being implicated as hormone disruptors, there also exists the mechanism for bromine substituting for the methyl group in the nitrogenous base 5-methyluracil of DNA, creating the base-analog 5-bromouracil, which can be incorporated into DNA and induce a point mutation via base substitution.[16]}
Metabolism
MDPV undergoes CYP450 2D6, 2C19, 1A2,[17] and COMT phase 1 metabolism (liver) into methylcatechol and pyrrolidine, which in turn are glucuronated (uridine 5'-diphospho-glucuronosyl-transferase) allowing it to be excreted by the kidneys, with only a small fraction of the metabolites being excreted into the stools.[18] No free pyrrolidine will be detected in the urine.[19]
Molecularly, this is seen as demethylenation of methylenedioxypyrovalerone (CYP2D6), followed by methylation of the aromatic ring via catechol-O-methyl transferase. Then hydroxylation of both the aromatic ring and side chain takes place followed by and oxidation of the pyrrolidine ring to the corresponding lactam, with subsequent detachment and ring opening to the corresponding carboxylic acid.[20]
Detection in biological specimens
MDPV may be quantitated in blood, plasma or urine by gas chromatography-mass spectrometry or liquid chromatography-mass spectrometry to confirm a diagnosis of poisoning in hospitalized patients or to provide evidence in a medicolegal death investigation. Blood or plasma MDPV concentrations are expected to be in a range of 10–50 μg/L in persons using the drug recreationally, >50 μg/L in intoxicated patients and >300 μg/L in victims of acute overdosage.[21]
Legality
In the UK, following the ACMD's report on substituted cathinone derivatives,[14] MDPV is a Class B drug under The Misuse of Drugs Act 1971 (Amendment) Order 2010, making it illegal to sell, buy, or possess without a license.[22][23] Penalties include a maximum of five years and/or unlimited fine for possession; up to 14 years and/or unlimited fine for production or trafficking. See list of drugs illegal in the UK for more information.
MDPV is specifically listed as a controlled substance in Finland (listed appendix IV substance as of June 28, 2010),[24] Denmark and Sweden. In Sweden a 33-year-old man has been sentenced to six years in prison by an appellate court, Hovrätt, for possession of 250 grams of MDPV that had been acquired prior to criminalization.[25]
Australia
In Western Australia, MDPV has been banned under the Poisons Act 1964, having been included in Appendix A Schedule 9 of the Poisons Act 1964 as from February 11, 2012. The Director of Public Prosecutions for Western Australia announced that anyone intending to sell or supply MDPV faces a maximum $100,000 fine or 25 years in jail. Users face a $2000 fine or two years' jail. So anyone caught with MDPV can be charged with possession, selling, supplying or intent to sell or supply.[26]
Canada
Canadian Health Minister Leona Aglukkaq announced on June 5, 2012 that MDPV would be listed on Schedule 1 of the Controlled Drugs and Substances Act. Other drugs on schedule 1 include cocaine and heroin. This will make possession, trafficking, importing, exporting, and production of MDPV illegal. As of September 26, 2012 MDPV has become illegal in Canada as a Schedule 1 drug. Researchers will still be able to use the drug after applying for an exemption to that status.[27]
United States
In the United States, MDPV is a DEA federally scheduled drug. On October 21, 2011, the DEA issued a temporary one-year ban on MDPV, classifying it as a schedule I substance. Schedule I status is reserved for those substances with a high potential for abuse, no currently accepted use for treatment in the United States and a lack of accepted safety for use of the drug under medical supervision.[28][29]
Prior to the Federal ban being announced, it was already banned in Louisiana and Florida.[30][31][32][33] On March 24, 2011, Kentucky passed bill HB 121 which makes MDPV, as well as three other cathinones, controlled substances in the state. It also makes it a Class A misdemeanor to sell the drug, and a Class B misdemeanor to possess it.[34]
MDPV is banned in New Jersey under Pamela's Law. The law is named after Pamela Schmidt, a Rutgers University student, murdered in March 2011 by an alleged user of MDPV.[35] A toxicology report later found that no "bath salts" were present in his system.[36]
On May 5, 2011, Tennessee Governor Bill Haslam signed a law making it a crime "to knowingly produce, manufacture, distribute, sell, offer for sale or possess with intent to produce, manufacture, distribute, sell, or offer for sale" any product containing 3,4-Methylenedioxypyrovalerone (MDPV).[37]
On July 6, 2011, the governor of Maine signed a bill establishing fines for possession and penalties for trafficking of MDPV.[38]
On September 7, 2011, the DEA took advantage of its emergency scheduling authority to ban Mephedrone (4-MMC), MDPV, and Methylone (M1). The substances were made illegal to possess and sell for 12 months until the DEA and Department of Health and Human Services determines if these substances should be permanently banned.[citation needed]
On October 17, 2011, an Ohio law banning synthetic drugs took effect barring selling and/or possession of "any material, compound, mixture, or preparation that contains any quantity of the following substances having a stimulant effect on the central nervous system, including their salts, isomers, and salts of isomers" listing ephedrine and pyrovalerone. It also specifically includes MDPV, misspelling the full name as "methyenedioxypyrovalerone".[39][40] Four days after this Ohio law was passed, the DEA's national emergency ban was implemented.[28]
On December 8, 2011, under the Synthetic Drug Control Act, the US House of Representatives voted to ban MDPV and a variety of other synthetic drugs which had been sold legally in stores.[41]
Documented misuse
In April 2011, two weeks after they went missing, two men in northwestern Pennsylvania were found dead in a remote location on government land. The official cause of death of both men was hypothermia, but toxicology reports later confirmed that both Troy Johnson, 29, and Terry Sumrow, 28, had ingested MDPV shortly before their deaths. "It wasn't anything to kill them, but enough to get them messed up," the county coroner said. MDPV containers were found in their vehicle along with spoons, hypodermic syringes and marijuana paraphernalia. In April 2011, an Alton, Illinois, woman apparently died from an MDPV overdose.[42] In May 2011, The CDC reported a hospital emergency department (ED) visit after the use of "bath salts" in Michigan. One person was reported dead on arrival at the ED. Associates of the dead person reported that he had used bath salts. His toxicology results revealed high levels of MDPV in addition to marijuana and prescription drugs. The primary factor contributing to death was cited as MDPV toxicity after autopsy was performed.[43] An incident of hemiplegia has been reported.[44]
Overdose treatment
Physicians often treat MDPV overdose cases with anxiolytics, such as benzodiazepines, to lessen the drug-induced activity in the brain and body.[45] In some cases, general anesthesia was used because sedatives were ineffective.[46]
Treatment in the emergency department for severe hypertension, tachycardia, agitation, or seizures consists of large doses of lorazepam in 2–4 mg increments every 10–15 minutes intravenously or intramuscularly. If this is not effective, haloperidol is an alternative treatment. It has been found that the use of any beta blockers to treat hypertension in these patients can cause an unopposed peripheral alpha-adrenergic effect with a dangerous paradoxical rise in blood pressure.[47]
Alternatively, the antihypertensive drug clonidine can be used to treat symptoms of MDPV intoxication. Clonidine has been used clinically in the treatment of acute stimulant intoxication and withdrawal,[48] and is sometimes prescribed to reduce the side effects of stimulant medications used to treat ADHD.[49] Some users of MDPV have reported a reduction in the severity of symptoms upon treatment with clonidine.[50][51][52]
References
- ↑ Simmler, L. D.; Buser, T. A.; Donzelli, M.; Schramm, Y.; Dieu, L-H.; Huwyler, J. et al. (2012). "Pharmacological characterization of designer cathinones in vitro". British Journal of Pharmacology 168 (2): 458. doi:10.1111/j.1476-5381.2012.02145.x. ISSN 0007-1188.
- ↑ Phenyl-2-pyrrolidino-Alkanones U.S. Patent 1-(3,4-Methylenedioxy Phenyl-2-pyrrolidino-Alkanones
- ↑ "Abuse of Fake 'Bath Salts' Sends Dozens To ER". KMBC.com. December 23, 2010.
- ↑ "MDPV Bath Salts Drug Over The Counter". Healthybodydaily.com. March 8, 2011. Archived from the original on 2011-03-10.
- ↑ Samantha Morgan (November 9, 2010). "Parents cautioned against over the counter synthetic speed". NBC 33 News. Retrieved May 16, 2011.
- ↑ Kelsey Scram (January 6, 2011). "Bath Salts Used to Get High". NBC 33 News. Retrieved May 16, 2011.
- ↑ 7.0 7.1 7.2 Brandt, S. D.; Freeman, S.; Sumnall, H. R.; Measham, F.; Cole, J. (2011). "Analysis of NRG 'legal highs' in the UK: Identification and formation of novel cathinones". Drug Testing and Analysis 3 (9): 569–75. doi:10.1002/dta.204. PMID 21960541.
- ↑ Westphal, F.; Junge, T.; Rösner, P.; Sönnichsen, F.; Schuster, F. (2009). "Mass and NMR spectroscopic characterization of 3,4-methylenedioxypyrovalerone: A designer drug with α-pyrrolidinophenone structure". Forensic Science International 190 (1–3): 1–8. doi:10.1016/j.forsciint.2009.05.001. PMID 19500924.
- ↑ 1-[(3,4-Methylenedioxy)phenyl]-2-pyrrolidino-1-alkanones as stimulants. (Boehringer Ingelheim G.m.b.H.). Brit. (1969), 7 pp. CODEN: BRXXAA GB 1149366 19690423 Patent. Priority: DE 19650523. CAN 72:21608 AN 1970:21608 CAPLUS
- ↑ Coppola, M.; Mondola, R. (2012). "3,4-Methylenedioxypyrovalerone (MDPV): Chemistry, pharmacology and toxicology of a new designer drug of abuse marketed online". Toxicology Letters 208 (1): 12–5. doi:10.1016/j.toxlet.2011.10.002. PMID 22008731.
- ↑ Watterson, L. R.; Kufahl, P. R.; Nemirovsky, N. E.; Sewalia, K.; Grabenauer, M.; Thomas, B. F.; Marusich, J. A.; Wegner, S.; Olive, M. F. (2012). "Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV)". Addiction Biology: 10.1111/j.1369–1600.2012.00474.x. doi:10.1111/j.1369-1600.2012.00474.x. PMC 3473160. PMID 22784198.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 "Report on MDPV" (PDF). Drugs of Concern. DEA.
- ↑ Terdiman, Daniel (November 12, 2012). "Antivirus pioneer McAfee sought for questioning in murder case | Security & Privacy – CNET News". News.cnet.com. Retrieved February 20, 2013.
- ↑ 14.0 14.1 "Consideration of the Cathinones" (PDF). Advisory Council on the Misuse of Drugs. March 31, 2010. Archived from the original on 2011-06-17.
- ↑ Loo, Paul (June 3, 2010). "Convenient Synthesis and Spectroscopic Data of Methcathinone Analogs" (PDF). 4th Seminar of European Customs Chemists.
- ↑ Nester, E. W.; Anderson, D. G.; Roberts, C. E. Jr. (June 4, 2009). Loose Leaf Version of Microbiology: A Human Perspective. McGraw-Hill. ISBN 978-0-07-736647-6. Retrieved June 25, 2011.
- ↑ Kalapos, Miklós Péter (2011). "3,4-metilén-dioxi-pirovaleron- (MDPV-) epidémia?". Orvosi Hetilap 152 (50): 2010–9. doi:10.1556/OH.2011.29259. PMID 22112374.
- ↑ Strano-Rossi, S.; Cadwallader, A. B.; De La Torre, X.; Botrè, F. (2010). "Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MPDV) by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometry". Rapid Communications in Mass Spectrometry 24 (18): 2706–14. doi:10.1002/rcm.4692. PMID 20814976.
- ↑ Michaelis, W.; Russel, J. H.; Schindler, O. (1970). "Metabolism of pyrovalerone hydrochloride". Journal of Medicinal Chemistry 13 (3): 497–503. doi:10.1021/jm00297a036. PMID 5441133.
- ↑ Meyer, M. R.; Du, P.; Schuster, F.; Maurer, H. H. (2010). "Studies on the metabolism of the α-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS". Journal of Mass Spectrometry 45 (12): 1426–42. doi:10.1002/jms.1859. PMID 21053377.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 10th edition, Biomedical Publications, Seal Beach, CA, in preparation.
- ↑ "A change to the Misuse of Drugs Act 1971 : Control of mephedrone and other cathinone derivatives". Home Office. April 16, 2010. Retrieved November 19, 2012.
- ↑ "The Misuse of Drugs Act 1971 (Amendment) Order 2010". Crown. Retrieved November 19, 2012.
- ↑ Suomen valtioneuvosto (28 June 2010). "Finlex: huumausaineina pidettävistä aineista, valmisteista ja kasveista annetun valtioneuvoston asetuksen liitteen IV muuttamisesta". Oikeusministeriö (in Finnish). Oikeusministeriö. Retrieved January 25, 2011.
- ↑ "Hovrätten skärper straff i MDPV-dom". Norrköpings Tidningar (in Swedish). June 4, 2010. Retrieved June 12, 2010.
- ↑ http://media.mediamonitors.com.au/ArticlePresenter.aspx?GUID=01e77e98-b704-48ab-96bd-9cc941ac963b&ArticleID=133825793&output=pdfsearchable (Not available from IA, WebCite, or Archive.is)
- ↑ "Bath salts ingredient mdpv banned". CBC News. September 26, 2012. Retrieved January 24, 2014.
- ↑ 28.0 28.1 DEA Press release (Oct 21, 2011). "Chemicals Used in "Bath Salts" Now Under Federal Control and Regulation" (Press release). Retrieved October 22, 2011.
- ↑ Harris, Elizabeth (Oct 22, 2011). "D.E.A. Bans Chemicals Used in 'Bath Salts'". New York Times. Retrieved October 22, 2011.
- ↑ Rakow, Erica (Jan 26, 2011). "Florida makees sales and possession of bath salts illegal". WJHG-TV. Retrieved January 27, 2011.
- ↑ Williams, Tyana (Jan 6, 2011). "Livingston deputies begin seizing "bath salts". WAFB. Retrieved January 8, 2011.
- ↑ McConnaughey, Janet (December 23, 2010). "Drugs disguised as bath salts send users to ERs". WAFB. Associated Press. Retrieved January 8, 2011.
- ↑ Allen, Greg (February 8, 2011). "Florida Bans Cocaine-Like 'Bath Salts' Sold in Stores". NPR. Retrieved May 22, 2011.
- ↑ Beshear, Steve. {D5D7C9CB-DDE8-4581-A6A9-40DA3FC6E877} "Gov. Beshear signs law banning new synthetic drugs" (Press release). Commonwealth of Kentucky. Retrieved March 23, 2011.
- ↑ Rowe, Amy (September 2, 2011). "Governor bans bath salts after student's death". Daily Targum. Archived from the original on January 27, 2014. Retrieved January 27, 2014. "Gov. Chris Christie signed "Pamela's Law" into legislation last week, which will ban the sale, possession and use of bath salts, a synthetic drug that affects users in a similar way to methamphetamines, in New Jersey. The law is named after Pamela Schmidt, a University student who was murdered in March. Authorities believe her boyfriend William Parisio Jr., who was under the influence of bath salts at the time of her murder, to be the suspect. ..."
- ↑ David, Giambusso (September 3, 2011). "Cranford man charged with murdering girlfriend; Toxicology report shows no trace of 'bath salts'". Nj.com. The Star-Ledger. Retrieved 27 January 2014. "... Parisio's toxicology report revealed no traces of the controversial "bath salts," thought by Parisio's mother to have played a role in the March 13 killing."
- ↑ "HOUSE BILL NO. 457, PUBLIC CHAPTER NO. 169. 39-17-452" (PDF). State of Tennessee. 2011. Retrieved January 24, 2014.
- ↑ "New law sets fine at $350 for 'bath salts' possession". Portland Press Herald. July 7, 2011. Retrieved July 7, 2011.
- ↑ "Ohio Amendment to Controlled Substances Act HB 64". Retrieved January 24, 2014.
- ↑ "Synthetic Drugs Ban Goes into effect". WBNS-TV Columbus. October 17, 2011.
- ↑ Kreider, Randy (December 8, 2011). "House Votes to Ban 'Spice,' 'Bath Salts'". ABC News. Retrieved May 30, 2012.
- ↑ Wilson, Todd (May 12, 2011). "Illinois lawmakers target bath salts used as a drug". Chicago Tribune. Retrieved May 22, 2011.
- ↑ "Emergency Department Visits After Use of a Drug Sold as 'Bath Salts' — Michigan, November 13, 2010 – March 31, 2011". Morbidity and Mortality Weekly Report 60 (19) (Centers for Disease Control and Prevention (CDC)). 2011. pp. 624–7. PMID 21597456.
- ↑ Boshuisen, K.; Arends, J. E.; Rutgers, D. R.; Frijns, C. J. (May 8, 2012) [published ahead of print April 25, 2012]. "A young man with hemiplegia after inhaling the bath salt "Ivory Wave."". Neurology 78 (19): 1533–1534. doi:10.1212/WNL.0b013e3182553c70. PMID 22539576.
- ↑ Salter, Jim; Suhr, Jim (April 7, 2011). "AP IMPACT: Synthetic drugs send thousands to ER". Bloomberg Businessweek. Retrieved May 22, 2011.
- ↑ Goodnough, Abby; Zezima, Katie (July 16, 2011). "An Alarming New Stimulant, Sold Legally in Many States". New York Times. Retrieved January 24, 2014.
- ↑ "Bath Salts Healthcare Provider Fact Sheet" (PDF). Feb 3, 2011.
- ↑ Marc Galanter; Herbert D Kleber, eds. (May 15, 1999). "Treatment of Acute Intoxication and Withdrawal from Drugs of Abuse" (PDF). Retrieved June 28, 2013. (Adapted or excerpted from: Marc Galanter; Herbert D. Kleber, eds. (1999). The Textbook of Substance Abuse Treatment (2nd ed.). The Press. ISBN 978-0-88048-820-4.)
- ↑ DeepDiveAdmin [screenname] (April 20, 2011). "Clonidine (Catapres)". Psyweb.com. Retrieved June 28, 2013.
- ↑ Meyer, Derek (September 27, 2012). "N-Pyrrolidinyl-Cathinones". Retrieved June 28, 2013.
- ↑ Luciano [screenname] (January 17, 2010). "MDPV? Comments/Opinion?". Hipforums.com. Retrieved June 28, 2013.
- ↑ Muie [screenname] (May 13, 2010). "Trying to Understand MDPV Comedown and Anxiety". Bluelight.ru. Retrieved June 28, 2013.
External links
| Look up methylenedioxypyrovalerone or MDPV in Wiktionary, the free dictionary. |
- Pubchem – similar compounds
-
 Meltzer, Peter C.; Butler, David; Deschamps, Jeffrey R.; Madras, Bertha K. (2006). "1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) Analogues: A Promising Class of Monoamine Uptake Inhibitors". Journal of Medicinal Chemistry 49 (4): 1420–32. doi:10.1021/jm050797a. PMC 2602954. PMID 16480278.
Meltzer, Peter C.; Butler, David; Deschamps, Jeffrey R.; Madras, Bertha K. (2006). "1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) Analogues: A Promising Class of Monoamine Uptake Inhibitors". Journal of Medicinal Chemistry 49 (4): 1420–32. doi:10.1021/jm050797a. PMC 2602954. PMID 16480278. - Erowid MDPV Vault
- ChemSub Online: Methylenedioxypyrovalerone.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||