2-Ethylhexanoic acid
| 2-Ethylhexanoic acid | |
|---|---|
 | |
 | |
| 2-Ethylhexanoic acid[3] | |
| Identifiers | |
| CAS number | 149-57-5 |
| PubChem | 8697, 78052 R, 135309 S |
| ChemSpider | 8373 |
| UNII | 01MU2J7VVZ |
| EC number | 205-743-6 |
| MeSH | 2-ethylhexanoic+acid |
| ChEMBL | CHEMBL1162485 |
| RTECS number | MO7700000 |
| Beilstein Reference | 1750468 |
| Jmol-3D images | {{#if:CCCCC(CC)c(:[o]):[oH]CCCCC(CC)C(O)=O|Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | C8H16O2 |
| Molar mass | 144.21 g mol−1 |
| Appearance | Colorless liquid |
| Density | 903 mg mL−1 |
| Melting point | −59.00 °C; −74.20 °F; 214.15 K |
| Boiling point | 228.1 °C; 442.5 °F; 501.2 K |
| log P | 2.579 |
| Vapor pressure | <1 Pa (at 25 °C) |
| Acidity (pKa) | 4.819 |
| Basicity (pKb) | 9.178 |
| Refractive index (nD) | 1.425 |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−635.1 kJ mol-1 |
| Std enthalpy of combustion ΔcH |
-4.8013–4.7979 MJ mol-1 |
| Hazards | |
| GHS pictograms |    |
| GHS signal word | DANGER |
| GHS hazard statements | H312, H318, H361 |
| GHS precautionary statements | P280, P305+351+338 |
| EU Index | 607-230-00-6 |
| EU classification | |
| R-phrases | R20/21/22, R37/38, R41, R63 |
| S-phrases | (S2), S26, S36/37/39 |
| Flash point | 114 °C; 237 °F; 387 K |
| Autoignition temperature | 371 °C; 700 °F; 644 K |
| Explosive limits | 0.9–6.7% |
| LD50 |
|
| Related compounds | |
| Related compounds | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
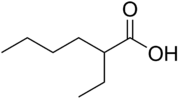
2-Ethylhexanoic acid is the organic compound with the formula CH3(CH2)3CH(C2H5)CO2H. This carboxylic acid is widely used to prepare metal derivatives that are soluble in nonpolar organic solvents. These lipophilic metal-containing derivatives are used as catalysts in polymerizations. For example, tin 2-ethylhexanoate is used in the production of poly(lactic-co-glycolic acid). The high solubility of these metal complexes is attributable to the long hydrocarbon chain and the presence of a chiral center which leads to mixtures of enantiomeric complexes. These metal complexes are often described as salts, when in fact they are not ionic but charge-neutral coordination complexes akin to the better defined, more crystalline acetates.
Examples of metal ethylhexanoates
- Hydroxyl aluminium bis(2-ethylhexanoate), used as a thickener
- Tin(II) ethylhexanoate (CAS# 301-10-0), a catalyst for polylactide polymerization
- Cobalt(II) ethylhexanoate (CAS# 136-52-7), a drier for alkyd resins
- Nickel(II) ethylhexanoate (CAS# 4454-16-4)
Health aspects
Some studies showed now subchronic oral toxicity.[4] but as a study indicated the teratogenicity of the compound the sources for exposure were evaluated.[5] One major source are the metal derivatives of 2-ethylhexanoic acid, which are widely used as stabilizers for polyvinyl chloride PVC. The other source is the metabolism of bis(2-ethylhexyl) phthalate (DEHP) the two ester bonds are hydrolysed and the resulting 2-ethylhexanol is oxidized in the organism to 2-ethylhexanoic acid. The elevated levels of the compound even in glass jars was due to the diffusion of the compound from the PVC cap into the food. [6]
References
- ↑ content1
- ↑ content2
- ↑ "2-ethylhexanoic acid - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 21 February 2012.
- ↑ Juberg DR, David RM, Katz GV, Bernard LG, Gordon DR, Vlaovic MS, Topping DC. (1998). "2-Ethylhexanoic acid: subchronic oral toxicity studies in the rat and mouse.". Food Chem Toxicol. 36 (5): 429–36. doi:10.1016/S0278-6915(97)00168-3. PMID 9662418.
- ↑ Ritter, E. J., Scott, W. J. Jr, Randall, J. L. and Ritter, J.M. (1987). "Teratogenicity of di(2-ethylhexyl)phthalate, 2-ethylhexanol, 2-ethylhexanoic acid, and valproic acid, and potentiation by caffeine.". Teratology 35 (1): 41–46. doi:10.1002/tera.1420350107. PMID 3105103.
- ↑ S. Elss; L. Grünewald; E. Richling; P. Schreier (2004). "Occurrence of 2-ethylhexanoic acid in foods packed in glass jars". Food Additives & Contaminants 21 (8): 811–814. doi:10.1080/02652030410001732879. PMID 15370833.