2,5-Furandicarboxylic acid
| 2,5-Furandicarboxylic acid | |
|---|---|
 | |
| Furan-2,5-dicarboxylic acid | |
| Other names Dehydromucic acid | |
| Identifiers | |
| CAS number | 3238-40-2 |
| PubChem | 76720 |
| ChemSpider | 69178 |
| Jmol-3D images | Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | C6H4O5 |
| Molar mass | 156.09 g mol−1 |
| Appearance | White solid |
| Density | 1.604 g/cm3 |
| Melting point | 342 °C |
| Boiling point | 420 °C |
| Solubility in water | soluble in DMSO |
| Acidity (pKa) | 2,28 |
| Hazards | |
| Flash point | 207 °C; 405 °F; 480 K |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
2,5-Furandicarboxylic acid (FDCA), also known as dehydromucic acid, is an oxidized furan derivative. This organic compound was first obtained by Fittig and Heinzelmann in 1876.[1] The first review by Henry Hill was already published in 1901.[2] FDCA has also been detected in human urine.[3] A healthy human produces 3–5 mg/day. Numerous studies were undertaken to establish the metabolism of this compound and to determine the quantity, which is produced depending on the healthiness of the human. It was demonstrated that the individual quantity of produced FDCA increased after the injection of fructose. FDCA was also detected in blood plasma.[4] FDCA was more than 125 years later identified by the US Department of Energy as one of 12 priority chemicals for establishing the “green” chemistry industry of the future. Furan-2,5-dicarboxylic acid (FDCA) has been suggested as an important renewable building block because it can substitute for terephthalic acid (PTA) in the production of polyesters and other current polymers containing an aromatic moiety.[4][5][6] Avantium has announced plans to produce 40 ton/year of FDCA monomer at a pilot plant in the second half of 2011.[7]
Synthesis of FDCA
Methods for the synthesis of the FDCA may be divided into four groups:[4]
- Dehydration of hexose derivatives
- Oxidation of 2,5-disubstituted furans
- Catalytic conversions of various furan derivatives
- Biological conversion of HMF
Dehydration of hexose derivatives
First group is based on the acid-promoted triple dehydration of aldaric (mucic) acids. This reaction requires severe conditions (highly concentrated acids, temp > 120 °C, React time > 20h) and all the methods were non-selective with yields < 50%.[1][8] The process has also been patented by the French company Agro Industrie Recherches et Developements.[9]
Oxidation of 2,5-disubstitured furans
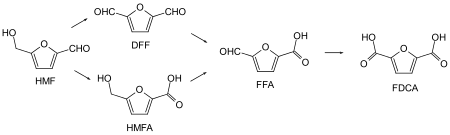
Catalytic conversions of various furan derivatives
The third class includes reactions describing the synthesis of FDCA from furfural. Furfural can be oxidized to 2-furoic acid with nitric acid and the latter was subsequently converted to its methyl ester. The ester was then converted via chloromethylation at position 5 to give 5-chloromethylfuroate. The latter was oxidized with nitric acid to form dimethyl 2,5-furandicarboxylate, which, after the alkaline hydrolysis gave FDCA in 50% yield. Andrisano reported that potassium 2-furoate, when heated up to 300 °C in a nitrogen atmosphere, underwent decarboxylation to furan with simultaneous carboxylation at position 5 to di-potassium 2,5-furandicarboxylate.[14]
Biological conversion of HMF
Recently, the enzyme furfural/HMF oxidoreductase was isolated from the bacterium Cupriavidus basilensis HMF14.[15] This enzyme is able to convert HMF to FDCA using molecular oxygen. A Pseudomonas putida strain that was genetically engineered to express this enzyme can completely and selectively convert HMF to FDCA. This biocatalysis is performed in water, at ambient temperature and pressure, without toxic or polluting chemicals, making it very environmentally friendly.[16]
History
In 1876 Fitting and Heinzelman synthesized FDCA from mucic acid using concentrated hydrobromic acid.[1] The first review by Henry Hill was already published in 1901.[2]
Properties and conversions
FDCA is a very stable compound. Its physical properties, such as insolubility in most of common solvents and a very high melting point (it melts at 342 °C) seem to indicate intermolecular hydrogen bonding. Despite its chemical stability, FDCA undergoes reactions typical for carboxylic acids, such as halogen substitution to give carboxylic dihalides, the di-ester formation and the formation of amides.[4] All these reactions were elaborated at the end of 19th and in the beginning of 20th century. Newer methods have been described by Janda et al., who introduced the synthesis of 2,5-furandicarboxylic dichloride, by the reaction of FDCA with thionyl chloride[17] The synthesis of diethyl ester and dimethyl ester as well as the amidation as well as several other modifications have been reported.[4] The versatility of FDCA is also seen in the number of derivatives available via relatively simple chemical transformations. Selective reduction can lead to partially hydrogenated products, such as 2,5 dihydroxymethylfuran, and fully hydrogenated materials, such as 2,5 bis(hydroxymethyl)tetrahydrofuran (Figure 2).Applications
The most important group of FDCA conversions is undoubtedly the polymerization. The potential applications of furan based building blocks for polymer applications has been extensively reviewed by Gandini.[18] Examples of polyesters, polyamides and polyurethanes have been described in literature. The company Avantium claims to have developed a cost effective route to produce FDCA and the derived polyesters via so-called YXY Building Blocks. FDCA has also been applied in pharmacology. It was demonstrated that its diethyl ester had a strong anaesthetic action similar to cocaine. Dicalcium 2,5-furandicarboxylate was shown to inhibit the growth of Baccillus megatorium. Screening studies on FDCA-derived anilides showed their important anti-bacterial action. The diacid itself is a strong complexing agent, chelating such ions as: Ca2+, Cu2+ and Pb2+, it is utilized in medicine to remove kidney stones.[4] Interestingly, HMF is metabolized via FDCA in mammals including humans.[1] A very diluted solution of FDCA in tetrahydrofuran is utilized for preparing artificial veins for transplantation. At the beginning of this chapter, it was mentioned that FDCA is a chemically stable compound. This property has been well benefited in industry – FDCA as most of polycarboxylic acids can be an ingredient of fire foams. Such foams help to extinguish fires in a short time caused by polar and non-polar solvents.[4]
Technical barriers
The primary technical barrier in the production and use of FDCA is the development of an effective and selective dehydration process from sugars. The control of sugar dehydration could be a very powerful technology, leading to a wide range of additional, inexpensive building blocks, but it is not yet well understood. Currently, dehydration processes using hydroxymethylfurfural (HMF) as intermediate are generally non-selective, unless, immediately upon their formation, the unstable intermediate products can be transformed into more stable materials such as methoxymethylfurfural (MMF). Necessary R&D will include development of selective dehydration systems and catalysts. FDCA formation will require development of cost effective and industrially viable oxidation technology that can operate in concert with the necessary dehydration processes.[5]
References
- ↑ 1.0 1.1 1.2 1.3 van Putten, R-J., van der Waal J.C. de Jong, E., Rasrendra C.B., Heeres, E.J. and de Vries H.G. (2011) Furan-based platform chemicals of the future. Dehydration of hexoses as biosustainable product route. Chemical Reviews submitted.
- ↑ 2.0 2.1 Hill 1901 Am. Chem. Journ. 25, 439
- ↑ Witten, T.A.; S.P. Levine, M. Killan, P. Boyle and S. Harkey. Clin. Chem. 1973, 19, 963
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Lewkowski J. 2001, Synthesis, chemistry and applications of 5-hydroxymethyl-furfural and its derivatives. ARKIVOC pp. 17-54
- ↑ 5.0 5.1 T. Werpy, G. Petersen: Top Value Added Chemicals from Biomass. Volume I – Results of Screening for Potential Candidates from Sugars and Synthesis Gas. Produced by the Staff at Pacific Northwest National Laboratory (PNNL); National Renewable Energy Laboratory (NREL), Office of Biomass Program (EERE), 2004 (Download)
- ↑ Bozell JJ, Petersen Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem 2010;12:539–554
- ↑
- ↑ Y. Taguchi, A. Oishi and H. Iida, Chem. Lett., 2008, 37, 50– 51
- ↑ ARD, FR2723945
- ↑ P. Verdeguer, N. Merat and A. Gaset (1993). "Oxydation catalytique du HMF en acide 2,5-furane dicarboxylique". Journal of Molecular Catalysis 85: 327–344. doi:10.1016/0304-5102(93)80059-4.
- ↑ Sara E. Davisa, Levi R. Houkb, Erin C. Tamargoa, Abhaya K. Datyeb and Robert J. Davisa (2 February 2011). "Oxidation of 5-hydroxymethylfurfuralnext term over supported Pt, Pd and Au catalysts". Catalysis Today 160 (1): 55–60. doi:10.1016/j.cattod.2010.06.004.
- ↑ W. Partenheimer and V.V. Grushin, Adv. Synth. Catal., 2001, 343, 102–111.
- ↑ C. Carlini, P. Patrono, A.M.R. Galletti, G. Sbrana and V. Zima, Appl. Catal., A, 2005, 289, 197–204; M.L. Ribeiro and U. Schuchardt, Catal. Commun., 2003, 4, 83– 86.
- ↑ Andrisano, R.; Angeloni, A.S. Ann. Chim. (Rome) 1963, 53, 1658
- ↑ F. Koopman, N. Wierckx, J.H. de Winde and H.J. Ruijssenaars. Proc. Nat. Acad. Sci. USA. 2010, 107: 4919-4924.
- ↑ F. Koopman, N. Wierckx, J.H. de Winde and H.J. Ruijssenaars. Bioresource Technology 2010, 101: 6291-6296.
- ↑ Janda, M.; Valenta, H.; Hrdy, I.; Hurkova, J.; Strogl, J.; Stibor, J.; Holy, P.; Bartizal, J. CS Patent, 188,011 (1982); C.A. 1982, 97, p72244h.
- ↑ Gandini, A., Belgacem, N.M. Prog. Polym. Sci., 1997, 22, 1203-1379; Gandini, A., Silvestre, A.J.D., Pascoal Neto, C. Sousa, A.F., Gomes, M. J. Pol. Sci.: Part A: Pol. Chem., 2009, 47, 295–298; Gandini, A. Pol. Chem. 1, 245-251.
