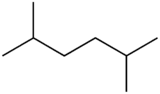
2,5-Dimethylhexane
From Wikipedia, the free encyclopedia
| 2,5-Dimethylhexane | |
|---|---|
 | |
 | |
| 2,5-Dimethylhexane[1] | |
| Identifiers | |
| CAS number | 592-13-2 |
| PubChem | 11592 |
| ChemSpider | 11104 |
| EC number | 209-745-8 |
| UN number | 3295 |
| Beilstein Reference | 1696877 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C8H18 |
| Molar mass | 114.23 g mol−1 |
| Appearance | Colourless liquid |
| Odor | Odourless |
| Density | 694 mg mL−1 |
| Melting point | −93 to −89 °C; −136 to −128 °F; 180 to 184 K |
| Boiling point | 108.1 to 109.9 °C; 226.5 to 229.7 °F; 381.2 to 383.0 K |
| Vapor pressure | 7.582 kPa (at 37.7 °C) |
| kH | 3.0 nmol Pa−1 kg−1 |
| Refractive index (nD) | 1.392 |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−262.0–−259.0 kJ mol−1 |
| Std enthalpy of combustion ΔcH |
−5.4615–−5.4587 MJ mol−1 |
| Specific heat capacity, C | 249.20 J K−1 mol−1 |
| Hazards | |
| GHS pictograms |     |
| GHS signal word | DANGER |
| GHS hazard statements | H225, H304, H315, H336, H410 |
| GHS precautionary statements | P210, P261, P273, P301+310, P331 |
| EU Index | 601-009-00-8 |
| EU classification | |
| R-phrases | R10, R38, R65, R67, R50/53 |
| S-phrases | (S2), S16, S29, S33 |
| Flash point | 26 °C; 79 °F; 299 K |
| Explosive limits | 0.98–?% |
| Related compounds | |
| Related alkanes | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
2,5-Dimethylhexane is a branched alkane used in the aviation industry in low revolutions per minute helicopters.[citation needed] As an isomer of octane, the boiling point is very close to that of octane, but can in pure form be slightly lower. 2,5-Dimethylhexane is moderately toxic.
References
- ↑ "2,5-DIMETHYLHEXANE - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 12 March 2012.
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.