2,5-Dimethoxy-4-nitroamphetamine
| 2,5-Dimethoxy-4-nitroamphetamine | |
|---|---|
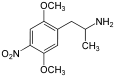 | |
| 1-(2,5-Dimethoxy-4-nitrophenyl)propan-2-amine | |
| Identifiers | |
| CAS number | 67460-68-8 |
| ChemSpider | 95083 |
| ChEMBL | CHEMBL8301 |
| Jmol-3D images | {{#if:COc1cc(c(cc1CC(C)N)OC)N(=O)=O[O-][N+](=O)c1cc(OC)c(cc1OC)CC(N)C|Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | C11H16N2O4 |
| Molar mass | 240.26 g mol−1 |
| Melting point | 206-207 °C (hydrochloride) 231-232 °C ((R)-isomer) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
2,5-Dimethoxy-4-nitroamphetamine (DON) is an analog of DOM and DOB. It is also closely related to 2C-N, and produces hallucinogenic, psychedelic, and entheogenic effects.
Chemistry
DON is in a class of compounds commonly known as alpha-methyl phenethylamines, or amphetamines and the full chemical name is 1-(2,5-dimethoxy-4-nitrophenyl)propan-2-amine. It has an active stereocenter and (R)-DON is the more active isomer. Concentrated solution is dark brown but transparent viscous liquid.
Effects
DON produces psychedelic and entheogenic effects that last up to 8–30 hours. In his book PiHKAL (Phenethylamines I Have Known And Loved), Alexander Shulgin lists a dosage of DON as being 3-4.5 mg orally. When dropped into nose, the dose is the same but onset is shorter. Dosages up to 7.5 mg reportedly produce high-intensity psychedelic effects with colorful visuals. There was a report of long-lasting psychosis in a young man living in Petrozavodsk, after taking 12.5 mg DON hydrochloride.[citation needed] The man accidentally used approximately 20 - 30 mg. There were no long-lasting physical complications. Unlike DOB, DON shows a visible stimulation effect, including muscular activity slightly similar to amphetamine. It also has been known to be more visual than most of its psychedelic amphetamine counterparts.
Pharmacology
The mechanism that produces the hallucinogenic and entheogenic effects of DON is similar to that of DOB and other analogs, this being agonist activity at a number of serotonin receptors, primarily 5-HT2A, 5-HT2C and 5-HT1A.
Dangers
The toxicity of DON is not known.
Legality
DON is unscheduled and unregulated in the United States, however because of its close similarity in structure and effects to DOM and DOB, possession and sale of DON may be subject to prosecution under the Federal Analog Act.[citation needed] DON is listed as a Class A drug in the Drugs controlled by the UK Misuse of Drugs Act after the table of contents of Pihkal and Tihkal were added to the schedules.
See also
References
External links
| ||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||