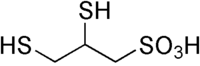
2,3-Dimercapto-1-propanesulfonic acid
| 2,3-Dimercapto-1-propanesulfonic acid | |
|---|---|
 | |
 | |
| Identifiers | |
| CAS number | 74-61-3 |
| PubChem | 6321 |
| ChemSpider | 6081 |
| KEGG | C10922 |
| MeSH | Unithiol |
| ChEBI | CHEBI:888 |
| Jmol-3D images | Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | C3H8O3S3 |
| Molar mass | 188.289 g/mol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
2,3-Dimercapto-1-propanesulfonic acid (abbreviated DMPS) and its sodium salt (known as Unithiol) are chelating agents that form complexes with various heavy metals. They are related to dimercaprol, which is another chelating agent.
The synthesis of DMPS was first reported in 1956 by V. E. Petrunkin.[1] The effects of DMPS on heavy metal poisoning, including with polonium-210, were investigated in the following years. DMPS was found to have some protective effect, prolonging the survival time.[2]
A study was undertaken of DMPS use by workers involved in the production of a calomel skin bleaching lotion and in direct contact with mercurous chloride and that already showed elevated urine mercury levels. The sodium salt of DMPS was found to be effective in lowering the body burden of mercury and in decreasing the urinary mercury concentration to normal levels.[3]
DMPS administrated to a mercury poisoned animal model failed to remove the mercury from tissues and reduce the inorganic mercury burden in the brain.[4][5] A 2008 study reported a case of Stevens–Johnson syndrome (SJS), a potentially serious disease, in a child undergoing chelation therapy with DMPS; the SJS resolved gradually after the chelation therapy was stopped.[6]
See also
References
- ↑ Petrunkin, V. E. (1956). "Synthesis and properties of dimercapto derivatives of alkylsulfonic acids". Ukrainsky Khemichisky Zhurnal 22: 603–607
- ↑ Aposhian, H.V.; Aposhian, M.M. (1990). "Meso-2,3-dimercaptosuccinic acid: Chemical, pharmacological and toxicological properties of an orally effective metal chelating agent.". Annual Review of Pharmacology and Toxicology 30 (1): 279–306. doi:10.1146/annurev.pa.30.040190.001431. PMID 2160791.
- ↑ D. Gonzalez-Ramirez, M. Zuniga-Charles, A. Narro-Juarez, Y. Molina-Recio, K. M. Hurlbut, R. C. Dart and H. V. Aposhian (1 October 1998). "DMPS (2,3-Dimercaptopropane-1-sulfonate, Dimaval) Decreases the Body Burden of Mercury in Humans Exposed to Mercurous Chloride" (free full text). Journal of Pharmacology and Experimental Therapeutics 287 (1): 8–12. PMID 9765315.
- ↑ Rooney, James (2007). "The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury". Toxicology 234 (3): 145–156. doi:10.1016/j.tox.2007.02.016. PMID 17408840.
- ↑ Guzzi, GianPaolo; Caterina A.M. La Porta (2008). "Molecular mechanisms triggered by mercury". Toxicology 244 (1): 1–12. doi:10.1016/j.tox.2007.11.002. PMID 18077077.
- ↑ Van der Linde AA, Pillen S, Gerrits GP, Bouwes Bavinck JN (2008). "Stevens–Johnson syndrome in a child with chronic mercury exposure and 2,3-dimercaptopropane-1-sulfonate (DMPS) therapy". Clin Toxicol (Phila) 46 (5): 479–81. doi:10.1080/15563650701779687. PMID 18568806.
| ||||||||||||||||||||||||||