2,3-Dimethylbutane
From Wikipedia, the free encyclopedia
| 2,3-Dimethylbutane | |
|---|---|
 | |
 | |
| 2,3-Dimethylbutane[1] | |
| Identifiers | |
| CAS number | 79-29-8 |
| PubChem | 6589 |
| ChemSpider | 6340 |
| EC number | 201-193-6 |
| UN number | 2457 |
| MeSH | 2,3-dimethylbutane |
| RTECS number | EJ9350000 |
| Beilstein Reference | 1730737 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C6H14 |
| Molar mass | 86.18 g mol−1 |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 662 mg mL−1 |
| Melting point | −136 to −124 °C; −213 to −191 °F; 137 to 149 K |
| Boiling point | 58.3 to 57.9 °C; 136.8 to 136.1 °F; 331.4 to 331.0 K |
| Vapor pressure | 26.1 kPa (at 21.1 °C) |
| kH | 7.6 nmol Pa−1 kg−1 |
| Refractive index (nD) | 1.375 |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−208.0–−206.0 kJ mol−1 |
| Std enthalpy of combustion ΔcH |
−4.1558–−4.1540 MJ mol−1 |
| Standard molar entropy S |
278.85 J K−1 mol−1 |
| Specific heat capacity, C | 189.02 J K−1 mol−1 |
| Hazards | |
| GHS pictograms |     |
| GHS signal word | DANGER |
| GHS hazard statements | H225, H304, H315, H336, H411 |
| GHS precautionary statements | P210, P261, P273, P301+310, P331 |
| EU Index | 601-007-00-7 |
| EU classification | |
| R-phrases | R11, R38, R65, R67, R51/53 |
| S-phrases | (S2), S16, S29, S33 |
| Flash point | −29 °C; −20 °F; 244 K |
| Autoignition temperature | 420 °C; 788 °F; 693 K |
| Explosive limits | 1.2–7.7% |
| Related compounds | |
| Related alkanes | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
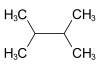
2,3-Dimethylbutane, also known as diisopropyl, is an isomer of hexane. It has the chemical formula (CH3)2CHCH(CH3)2 It is a colorless liquid which boils at 57.9 °C.
References
- ↑ "2,3-dimethylbutane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 10 March 2012.
| ||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.