1-Octen-3-ol
| 1-Octen-3-ol | |
|---|---|
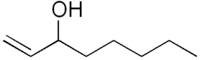 | |
| Oct-1-en-3-ol | |
| Other names Amyl vinyl carbinol; 1-Vinylhexanol; Matsutake alcohol; Vinyl amyl carbinol; Vinyl hexanol; Matsuica alcohol; Mushroom alcohol; 3-Hydroxy-1-octene | |
| Identifiers | |
| CAS number | 3391-86-4 |
| PubChem | 18827 |
| ChemSpider | 17778 |
| UNII | WXB511GE38 |
| KEGG | C14272 |
| ChEBI | CHEBI:34118 |
| Jmol-3D images | Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | C8H16O |
| Molar mass | 128.21 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
1-Octen-3-ol, octenol for short and also known as mushroom alcohol,[1] is a chemical that attracts biting insects such as mosquitos. It is contained in human breath and sweat, and it was once believed that insect repellent DEET works by blocking the insects' octenol odorant receptors.[2][3] 1-Octen-3-ol is a secondary alcohol derived from 1-octene. It exists in the form of two enantiomers, (R)-(–)-1-octen-3-ol and (S)-(+)-1-octen-3-ol.
Natural occurrence
Octenol is produced by several plants and fungi, including edible mushrooms and Lemon balm. Octenol is formed during oxidative breakdown of linoleic acid.[4]
Uses
Octenol is used, sometimes in combination with carbon dioxide, to attract insects in order to kill them with certain electronic devices.[5]
Its odor has been described as green and moldy or meaty; it is used in certain perfumes.[citation needed]
Health and safety
Octenol is approved by the U.S. Food and Drug Administration as a food additive.[6] It is of moderate toxicity with an LD 50 of 340 mg/kg.[5]
In an animal study, octenol has been found to disrupt dopamine homeostasis and may be an environmental agent involved in parkinsonism.[7]
See also
- Olfactory receptor
- Oct-1-en-3-one, the ketone analog that gives blood on skin its typical metallic, mushroom-like smell[citation needed]
References
- ↑ "1-Octen-3-ol, Mushroom alcohol, 3-Octenol, 3391-86-4". Retrieved 2008-11-14.
- ↑ Anna Petherick (2008-03-13). "How DEET jams insects' smell sensors". Nature News. Archived from the original on 15 March 2008. Retrieved 2008-03-16.
- ↑ Mathias Ditzen, Maurizio Pellegrino, Leslie B. Vosshall (2008). "Insect Odorant Receptors Are Molecular Targets of the Insect Repellent DEET". Sciencexpress 319 (5871): 1838–42. doi:10.1126/science.1153121. PMID 18339904.
- ↑ "Chemical properties of attractants". Retrieved 2010-06-08.
- ↑ 5.0 5.1 EPA fact sheet 1-Octen-3-ol
- ↑ US FDAs Center for Food Safety and Applied Nutrition. "US FDA/CFSAN - EAFUS List". Archived from the original on 21 February 2008. Retrieved 2008-03-16.
- ↑ Inamdar, A. A.; Hossain, M. M.; Bernstein, A. I.; Miller, G. W.; Richardson, J. R.; Bennett, J. W. (2013). "Fungal-derived semiochemical 1-octen-3-ol disrupts dopamine packaging and causes neurodegeneration". Proceedings of the National Academy of Sciences 110 (48): 19561. doi:10.1073/pnas.1318830110.