1,5-Diazabicyclo(4.3.0)non-5-ene
| 1,5-Diazabicyclo[4.3.0]non-5-ene | |
|---|---|
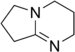 | |
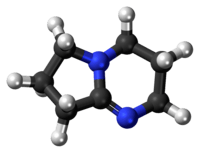 | |
| 2,3,4,6,7,8-hexahydropyrrolo[1,2-a]pyrimidine | |
| Other names DBN | |
| Identifiers | |
| CAS number | 3001-72-7 |
| ChemSpider | 68826 |
| Jmol-3D images | {{#if:C1CC2=NCCCN2C1[N-]=[N+]3C=1CCCC23CCCC=12|Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | C7H12N2 |
| Molar mass | 124.18 g/mol |
| Density | 1.005 g/cm³ |
| Boiling point | 95–98 °C at 7.5 mmHg |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
1,5-Diazabicyclo[4.3.0]non-5-ene (DBN) is a chemical compound with the formula C7H12N2.[1] It is an amidine base used in organic synthesis. A related compound with related functions is 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). The relatively complex nature of the formal names for DBU and DBN (hence the common use of acronyms) reflects the fact that these compounds are bicyclic and contain several functional groups.
The compounds are employed for dehydrohalogenation[2] reactions as well as base-catalyzed rearrangements.
References
- ↑ Savoca, Ann. C. "1,5-Diazabicyclo[4.3.0]non-5-ene" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.rd010.pub2
- ↑ Möller, Fr.; Oediger, H. "1,5-Diazabicyclo[5.4.0]undec-5-ene, a New Hydrogen Halide Acceptor" Angew. Chem., Int. Ed. Engl. , 1967, 5, 76. doi:10.1002/anie.196700761