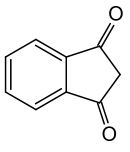
1,3-Indandione
| 1,3-Indandione | |
|---|---|
 | |
 | |
| indane-1,3-dione | |
| Other names Indandione; 1,3-Diketohydrindene; 1,3-Dioxoindane; 1,3-Hydrindendione | |
| Identifiers | |
| CAS number | 606-23-5 |
| PubChem | 11815 |
| ChemSpider | 11322 |
| ChEMBL | CHEMBL283521 |
| Jmol-3D images | Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | C9H6O2 |
| Molar mass | 146.14 g mol−1 |
| Appearance | Yellow solid |
| Density | 1.37 g / cm3 |
| Melting point | 129 to 132 °C; 264 to 270 °F; 402 to 405 K ([1][2]) |
| Solubility in water | slight |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
1,3-Indandione is an aromatic trans-fixed β-diketone. In standard conditions it is referred to in different sources as either colorless or yellowish,[3] green,[4] or (most commonly) yellow solid.
Structural properties
In the solid state, 1,3-indandione occurs as a diketone; in water, it is partially (~2%) enolized. The enolate anion exhibits significant delocalization, and the highest electron density is on the second carbon. This explains many of chemical properties of the compound.
Preparation
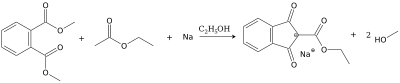
1,3-Indandione can be prepared by decarboxylation of the sodium salt of 2-etoxycarbonyl-1,3-indandione, which itself is obtained by Claisen condensation of ethyl acetate and dimethyl phthalate.
Chemical properties
1,3-Indandione is a very strong C-nucleophile. It undergoes self-condensation quite easily, resulting in bindone.
Bromination occurs at the 2-position:
1,3-Indandione could be reduced to indanone, 3-hydroxy-1-indanone, 1,3-indanediol or even indane, depending on the method used.
Uses
Certain derivatives are used in human medicine. Some other ones could be promising materials in the photonics field.
In addition, 1,3-indandione ("indanedione") is used in the first stage of forensic identification of latent finerprints. It is particularly useful for paper, and for items printed with thermal inks such as receipts. Amino acids left behind by the human hand may be developed into fingerprints by the use of it; the results, photographed with a special filter under a strong yellow-green fluorescent or green laser. It is usually the first method employed in a sequential analysis aimed at the production of evidence of a grade suitable for use in the courtroom. [5]
See also
References
- ↑ 1,3-Indandione at Sigma-Aldrich
- ↑ MSDS at Acros Organics, retrieved on June 16, 2011
- ↑ (Russian) Нейланд О. Я. Органическая химия: Учеб. для хим. спец. вузов. Москва: Высшая школа, 1990.— с. 481—490.
- ↑ Datapage, AlfaAesar, June 16, 2011
- ↑ "Sequential Processing 2010 : 01 : History of Indanedione". Sequential Processing of Documents For Fingerprints (NFSTC). Retrieved August 2, 2013.
| |||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||