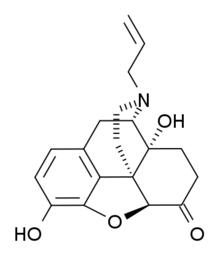
(+)-Naloxone
 | |
|---|---|
| Systematic (IUPAC) name | |
| (1R,5S,13S,17R)- 10,17-dihydroxy- 4-(prop-2-en-1-yl)- 12-oxa- 4-azapentacyclo [9.6.1.01,13.05,17.07,18] octadeca- 7(18),8,10-trien- 14-one | |
| Clinical data | |
| MedlinePlus | a601092 |
| Legal status | Uncontrolled |
| Identifiers | |
| ATC code | ? |
| PubChem | CID 5491858 |
| ChemSpider | 4590735 |
| Chemical data | |
| Formula | C19H21NO4 |
| Mol. mass | 327.374 g/mol |
| |
| |
| | |
(+)-Naloxone (dextro-naloxone) is a drug which is the "unnatural" enantiomer of the opioid antagonist drug (-)-naloxone. Unlike "normal" naloxone, (+)-naloxone has no significant affinity for opioid receptors,[1] but instead has been discovered to act as a selective antagonist of Toll-like receptor 4. This receptor is involved in immune system responses, and activation of TLR4 induces glial activation and release of inflammatory mediators such as TNF-α and Interleukin-1.[2][3]
Relation of TLR4 to opioid drugs
Both "normal" and "unnatural" enantiomers of various opioid analgesic drugs including morphine, meperidine, fentanyl, methadone and buprenorphine, as well as some otherwise inactive metabolites like morphine-3-glucuronide, have been found to act as agonists of TLR4, and chronic use of these drugs consequently causes constant low-level release of TNF-α and IL-1β as well as other downstream effects. This is thought to be involved in various adverse properties of opioid analgesic drugs, such as loss of efficacy with extended use and the associated development of tolerance and dependence, as well as the development of side effects such as hyperalgesia and allodynia, which can cause long-term use of opioid analgesics to not only fail to treat neuropathic pain, but ultimately exacerbate it.[4][5]
Applications of (+)-naloxone and related drugs
Several opioid antagonist drugs were found to act as antagonists for TLR4, including naloxone and naltrexone. However it was found that not only the "normal" (-) enantiomers, but also the "unnatural" (+) enantiomers of these drugs acted as TLR4 antagonists (though (+)-nalmefene was inactive). Since (+)-naloxone and (+)-naltrexone lack affinity for opioid receptors, they do not block the effects of opioid analgesic drugs, and so can be used to counteract the TLR4-mediated side effects of opioid agonists without affecting analgesia,[6] though (+)-naloxone does reduce the reinforcing effects of opioid drugs.[7] (+)-Naloxone was also found to be neuroprotective,[8][9] and both (+)-naloxone and (+)-naltrexone are effective in their own right at treating symptoms of neuropathic pain in animal models.[10][11] However (+)-naloxone was also found to reduce the effects of stimulant drugs,[12][13] suggesting additional actions beyond TLR4 antagonism (possibly as a sigma receptor antagonist),[14] that might potentially result in unwanted side effects or drug interactions.
See also
References
- ↑ Iijima, I.; Minamikawa, J.; Jacobson, A. E.; Brossi, A.; Rice, K. C.; Klee, W. A. (1978). "Studies in the (+)-morphinan series. 5. Synthesis and biological properties of (+)-naloxone". Journal of medicinal chemistry 21 (4): 398–400. doi:10.1021/jm00202a018. PMID 206698.
- ↑ Wu, Hsiang-En; Thompson, Jonathan; Sun, Han-Sen; Terashvili, Maia; Tseng, Leon F. (September 2005). "Antianalgesia: stereoselective action of dextro-morphine over levo-morphine on glia in the mouse spinal cord". The Journal of Pharmacology and Experimental Therapeutics 314 (3): 1101–8. doi:10.1124/jpet.105.087130. PMID 15901793.
- ↑ Watkins, Linda R.; Hutchinson, Mark R.; Rice, Kenner C.; Maier, Steven F. (2009). "The "Toll" of Opioid-Induced Glial Activation: Improving the Clinical Efficacy of Opioids by Targeting Glia". Trends in Pharmacological Sciences 30 (11): 581–591. doi:10.1016/j.tips.2009.08.002. PMC 2783351. PMID 19762094.
- ↑ Hutchinson, Mark R.; Zhang, Yingning; Shridhar, Mitesh; Evans, John H.; Buchanan, Madison M.; Zhao, Tina X.; Slivka, Peter F.; Coats, Benjamen D.; Rezvani, Niloofar; Wieseler, Julie; Hughes, Travis S.; Landgraf, Kyle E.; Chan, Stefanie; Fong, Stephanie; Phipps, Simon; Falke, Joseph J.; Leinwand, Leslie A.; Maier, Steven F.; Yin, Hang; Rice, Kenner C.; Watkins, Linda R. (2010). "Evidence that opioids may have toll-like receptor 4 and MD-2 effects". Brain, Behavior, and Immunity 24: 83–95. doi:10.1016/j.bbi.2009.08.004. PMC 2788078. PMID 19679181.
- ↑ Hutchinson, Mark R.; Lewis, S. S.; Coats, Benjamen D.; Rezvani, Niloofar; Zhang, Y.; Wieseler, Julie L.; Somogyi, A. A.; Yin, Hang; Maier, Steven F.; Rice, Kenner C.; Watkins, Linda R. (19 May 2010). "Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences". Neuroscience 167 (3): 880–893. doi:10.1016/j.neuroscience.2010.02.011. PMC 2854318. PMID 20178837.
- ↑ Wu, Hsiang-En; Sun, Han-Sen; Cheng, Caleb W.; Terashvili, Maia; Tseng, Leon F. (November 2006). "dextro-Naloxone or levo-naloxone reverses the attenuation of morphine antinociception induced by lipopolysaccharide in the mouse spinal cord via a non-opioid mechanism". The European Journal of Neuroscience 24 (9): 2575–2580. doi:10.1111/j.1460-9568.2006.05144.x. PMID 17100845.
- ↑ Hutchinson, M. R.; Northcutt, A. L.; Hiranita, T.; Wang, X.; Lewis, S. S.; Thomas, J.; Van Steeg, K.; Kopajtic, T. A.; Loram, L. C.; Sfregola, C.; Galer, E.; Miles, N. E.; Bland, S. T.; Amat, J.; Rozeske, R. R.; Maslanik, T.; Chapman, T. R.; Strand, K. A.; Fleshner, M.; Bachtell, R. K.; Somogyi, A. A.; Yin, H.; Katz, J. L.; Rice, K. C.; Maier, S. F.; Watkins, L. R. (15 August 2012). "Opioid Activation of Toll-Like Receptor 4 Contributes to Drug Reinforcement". Journal of Neuroscience 32 (33): 11187–11200. doi:10.1523/JNEUROSCI.0684-12.2012. PMC 3454463. PMID 22895704.
- ↑ Liu, Bin; Du, Lina; Hong, Jau-Shyong (1 May 2000). "Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation". The Journal of Pharmacology and Experimental Therapeutics 293 (2): 607–617. PMID 10773035.
- ↑ Liu, Yuxin; Qin, Liya; Wilson, Belinda C.; An, Lijia; Hong, Jau-Shyong; Liu, Bin (1 September 2002). "Inhibition by naloxone stereoisomers of β-amyloid peptide (1-42)-induced superoxide production in microglia and degeneration of cortical and mesencephalic neurons". The Journal of Pharmacology and Experimental Therapeutics 302 (3): 1212–1219. doi:10.1124/jpet.102.035956. PMID 12183682.
- ↑ Hutchinson, Mark R.; Zhang, Yingning; Brown, Kimberley; Coats, Benjamen D.; Shridhar, Mitesh; Sholar, Paige W.; Patel, Sonica J.; Crysdale, Nicole Y.; Harrison, Jacqueline A.; Maier, Steven F.; Rice, Kenner C.; Watkins, Linda R. (July 2008). "Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4)". European Journal of Neuroscience 28 (1): 20–29. doi:10.1111/j.1460-9568.2008.06321.x. PMC 2588470. PMID 18662331.
- ↑ Lewis, Susannah S.; Loram, Lisa C.; Hutchinson, Mark R.; Li, Chien-Ming; Zhang, Yingning; Maier, Steven F.; Huang, Yong; Rice, Kenner C.; Watkins, Linda R. (2012). "(+)-Naloxone, an Opioid-Inactive Toll-Like Receptor 4 Signaling Inhibitor, Reverses Multiple Models of Chronic Neuropathic Pain in Rats". The Journal of Pain 13 (5): 498–506. doi:10.1016/j.jpain.2012.02.005. PMC 3348259. PMID 22520687.
- ↑ Chatterjie, Nithiananda; Alexander, George J.; Sechzer, Jeri A.; Lieberman, Kenneth W. (June 1996). "Prevention of cocaine-induced hyperactivity by a naloxone isomer with no opiate antagonist activity". Neurochemical Research 21 (6): 691–3. doi:10.1007/BF02527726. PMID 8829141.
- ↑ Chatterjie, Nithiananda; Sechzer, Jeri A.; Lieberman, Kenneth W.; Alexander, George J. (February 1998). "Dextro-naloxone counteracts amphetamine-induced hyperactivity". Pharmacology, Biochemistry and Behavior 59 (2): 271–274. doi:10.1016/S0091-3057(97)00528-5. PMID 9476969.
- ↑ Wu, Hsiang-en; Hong, Jau-Shyong; Tseng, Leon F. (1 October 2007). "Stereoselective action of (+)-morphine over (−)-morphine in attenuating the (−)-morphine-produced antinociception via the naloxone-sensitive sigma receptor in the mouse". European Journal of Pharmacology 571 (2–3): 145–151. doi:10.1016/j.ejphar.2007.06.012. PMC 2080825. PMID 17617400.