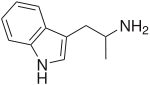
Alpha-Methyltryptamine
 | |
|---|---|
 | |
| Systematic (IUPAC) name | |
| 2-(1H-indol-3-yl)-1-methyl-ethylamine | |
| Clinical data | |
| Legal status | Controlled (S8) (AU) Uncontrolled (CA) Legal (UK) Schedule I (US) |
| Routes | Oral, Insufflation, Rectal, Smoked, IM, IV[1] |
| Identifiers | |
| CAS number | 299-26-3 |
| ATC code | None |
| PubChem | CID 9287 |
| DrugBank | DB01446 |
| ChemSpider | 8930 |
| ChEBI | CHEBI:59020 |
| ChEMBL | CHEMBL30713 |
| Synonyms | Indopan; IT-290, IT-403, U-14,164E, 3-IT[1] |
| Chemical data | |
| Formula | C11H14N2 |
| Mol. mass | 174.24 g/mol |
| SMILES
| |
| |
| | |
α-Methyltryptamine (αMT, AMT, Indopan) is a psychedelic, stimulant, and entactogen drug of the tryptamine class.[2][3] It was originally developed as an antidepressant by workers at Upjohn in the 1960s.[4]
Chemistry
αMT is tryptamine with a methyl substituent at the alpha carbon. Its chemical relation to tryptamine is analogous to that of amphetamine to phenethylamine, amphetamine being α-methylphenethylamine. αMT is closely related to the neurotransmitter serotonin (5-hydroxytryptamine) which partially explains its mechanism of action.
Pharmacology
αMT acts as a relatively balanced reuptake inhibitor and releasing agent of the main three monoamines; serotonin, norepinephrine, and dopamine,[5] and as a non-selective serotonin receptor agonist.[6]
MAOI activity
αMT has been shown as a reversible inhibitor of the enzyme monoamine oxidase (MAO) in-vitro[7] and in-vivo.[8]
In rats the potency of αMT as an MAO-A inhibitor in the brain was approximately equal to that of harmaline at equimolar doses.[note 1] Dexamphetamine did not enhance the 5-hydroxytryptophan-induced rise of serotonin at any level.[9]
A typical dose of harmaline for MAO inhibition is 150 mg,[10] higher than any typical αMT dose[11] so aMT's MAOI activity at typical doses will be significant but not total. The danger rises exponentially as αMT doses approach 150 mg due to increased monoamine release and increased MAO inhibition.
Metabolism
2-Oxo-AMT, 6-hydroxy-AMT,[12] 7-hydroxy-AMT and 1′-hydroxy-AMT were detected as metabolites of AMT in male Wistar rats.[13]
Dosage and effects
With 20–30 milligrams, euphoria, empathy, and psychedelic effects become apparent and can last as long as 12 hours.[14] A dose exceeding 40 mg is generally considered as strong. In rare cases or extreme doses the duration of effects might exceed 24 hours. αMT in freebase form is reported by users to have been smoked, with doses of between and 2–5 milligrams being cited.[1][2]
Reported side effects include anxiety, restlessness, muscle tension, jaw tightness, pupil dilation, tachycardia, headaches, nausea, and vomiting, among other effects that might commonly be attributed to LSD, psilocybin, DMT and MDMA, such as delusions and hallucinations.[2][15]
In spite of some reported experiential similarities to MDMA, the chemicals are structurally unrelated; αMT is a tryptamine while MDMA is a phenethylamine.
Like many other serotonin releasing agents, αMT's analogue αET has been shown to produce long-lasting serotonergic neurotoxicity at very high doses.[16] It is possible that αMT could cause the same neurotoxicity.
Legality
Australia
The 5-Methoxy analogue, 5-MeO-αMT is schedule 9 in Australia and αMT would be controlled as an analogue of this.[17]
Sweden
Sveriges riksdags health ministry Statens folkhälsoinstitut classified αMT as "health hazard" under the act Lagen om förbud mot vissa hälsofarliga varor (translated Act on the Prohibition of Certain Goods Dangerous to Health) as of Mar 1, 2005, in their regulation SFS 2005:26 listed as alfa-metyltryptamin (AMT), making it illegal to sell or possess.[18]
UK
It is legal in the United Kingdom, however, and does not fall under the tryptamine clause as its substituent is not on the nitrogen position. See "2001 Misuse of Drugs Act: Schedule 1, Regulation 3"[19] for more information. Similarly, Canada has no mention of this substance in the Controlled Drugs and Substances Act.[20]
USA
The Drug Enforcement Administration (DEA) placed MT temporarily in schedule I of the Controlled Substances Act (CSA) on April 4, 2003, pursuant to the temporary scheduling provisions of the CSA (68 FR16427). On September 29, 2004, AMT was permanently controlled as a schedule I substance under the CSA (69FR 58050).[21]
Reported Deaths
Deaths from αMT are rare but as a powerful monoamine releaser injury can occur when excessive doses are taken or when taken with drugs such as MAOIs.[22] Most fatalities are not verified but those which are involve excessive doses[23] or coingestion with other drugs.[24] A British teenager died after consuming 1 g of αMT in August 2013.[25]
See also
- 4-Methyl-αMT
- 5-Fluoro-αMT
- 5-API
- 5-MeO-αMT
- α-Ethyltryptamine
- α,N-DMT
- α,N,N-TMT
- α-Methyl-5-HT
Notes
References
- ↑ 1.0 1.1 1.2 "Erowid AMT Vault : FAQ by Dialtonez".
- ↑ 2.0 2.1 2.2 "Erowid Online Books : "TIHKAL" - #48 a-MT".
- ↑ "Erowid AMT (alpha-methyltryptamine) Vault".
- ↑ US Patent 3296072, Szmuszkovicz Jacob, "Method of Treating Mental Depression", published 1967-01-03, assigned to Upjohn Co
- ↑ Nagai F, Nonaka R, Satoh Hisashi Kamimura K (March 2007). "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology 559 (2-3): 132–7. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- ↑ Nonaka R, Nagai F, Ogata A, Satoh K (December 2007). "In vitro screening of psychoactive drugs by [(35)S]GTPgammaS binding in rat brain membranes". Biological & Pharmaceutical Bulletin 30 (12): 2328–33. PMID 18057721.
- ↑ Arai, Y.; Toyoshima, Y.; Kinemuchi, H. (1986). "Studies of monoamine oxidase and semicarbazide-sensitive amine oxidase. II. Inhibition by .ALPHA.-methylated substrate-analogue monoamines, .ALPHA.-methyltryptamine, .ALPHA.-methylbenzylamine and two enantiomers of .ALPHA.-methylbenzylamine". The Japanese Journal of Pharmacology 41 (2): 191. doi:10.1254/jjp.41.191.
- ↑ Greig, M. E.; Walk, R. A.; Gibbons, A. J. (1959). "The effect of three tryptamine derivatives on serotonin metabolism in vitro and in vivo". The Journal of pharmacology and experimental therapeutics 127: 110–115. PMID 13851725.
- ↑
- ↑ "Shulgin, A (1997) ''TIHKAL''". Erowid.org. Retrieved 2013-10-16.
- ↑ "AMT Dosage". Erowid. 2011-02-02. Retrieved 2013-10-16.
- ↑ Szara, S. (1961). "6-Hydroxylation: An important metabolic route for α-methyltryptamine". Experientia 17 (2): 76–76. doi:10.1007/BF02171429.
- ↑ Kanamori, T.; Kuwayama, K.; Tsujikawa, K.; Miyaguchi, H.; Iwata, Y. T.; Inoue, H. (2008). "In vivometabolism of α-methyltryptamine in rats: Identification of urinary metabolites". Xenobiotica 38 (12): 1476–1486. doi:10.1080/00498250802491654. PMID 18982537.
- ↑ Wilcox, J. (2012). "Psychoactive Properties of Alpha-Methyltryptamine: Analysis from Self Reports of Users". Journal of Psychoactive Drugs 44 (3): 274–276. doi:10.1080/02791072.2012.704592. PMID 23061328.
- ↑ "Erowid AMT Vault : Effects".
- ↑ Huang XM, Johnson MP, Nichols DE (July 1991). "Reduction in brain serotonin markers by alpha-ethyltryptamine (Monase)". European Journal of Pharmacology 200 (1): 187–90. doi:10.1016/0014-2999(91)90686-K. PMID 1722753.
- ↑ "Erowid 5-MeO-AMT Vault : Legal Status".
- ↑ "Förordning om ändring i förordningen (1999:58) om förbud mot vissa hälsofarliga varor;" (pdf), Lagen om förbud mot vissa hälsofarliga varor - SFS 2005:26 (in Swedish), February 2005, retrieved 2013-10-07
- ↑ 2001 Misuse of Drugs Act
- ↑ "CSDA".
- ↑ "ALPHA-METHYLTRYPTAMINE". DEA Office of Diversion Control. April 2013. Archived from the original on 2013-10-01. Retrieved 2013-10-10.
- ↑ Gillman, P. K. (2005). "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity". British Journal of Anaesthesia 95 (4): 434–441. doi:10.1093/bja/aei210. PMID 16051647. "drugs such as MDMA, ecstasy (3,4-methylenedioxymethamphetamine), if combined with MAOIs (including moclobemide) do also cause fatalities because they act as serotonin releasers"
- ↑ Boland, Diane M.; Andollo W, Hime GW, Hearn WL (July–August 2005). "Fatality Due to Acute Alpha-methyltryptamine Intoxication". Journal of Analytical Toxicology 29: 394–397. Retrieved October 1, 2013.
- ↑ "Call for ban on drug after reveller's death". Western Gazette. March 22, 2012. Archived from the original on 2013-10-16. Retrieved October 1, 2013.
- ↑ "Southampton 'legal high' death deemed 'accidental'". BBC News. 2013-11-12. Retrieved 2013-11-19.
External links
| ||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||