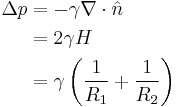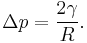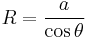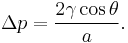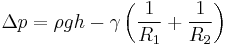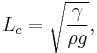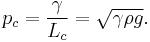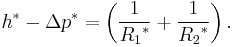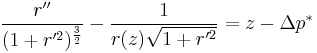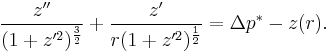Young–Laplace equation
In physics, the Young–Laplace equation is a nonlinear partial differential equation that describes the capillary pressure difference sustained across the interface between two static fluids, such as water and air, due to the phenomenon of surface tension or wall tension, although usage on the latter is only applicable if assuming that the wall is very thin. The Young–Laplace equation relates the pressure difference to the shape of the surface or wall and it is fundamentally important in the study of static capillary surfaces. It is a statement of normal stress balance for static fluids meeting at an interface, where the interface is treated as a surface (zero thickness):
where 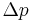 is the pressure difference across the fluid interface, γ is the surface tension (or wall tension),
is the pressure difference across the fluid interface, γ is the surface tension (or wall tension), 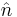 is the unit normal pointing out of the surface,
is the unit normal pointing out of the surface, 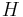 is the mean curvature, and
is the mean curvature, and 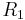 and
and 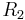 are the principal radii of curvature. (Some authors refer inappropriately to the factor
are the principal radii of curvature. (Some authors refer inappropriately to the factor 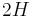 as the total curvature.) Note that only normal stress is considered, this is because it can be shown[1] that a static interface is possible only in the absence of tangential stress.
as the total curvature.) Note that only normal stress is considered, this is because it can be shown[1] that a static interface is possible only in the absence of tangential stress.
The equation is named after Thomas Young, who developed the qualitative theory of surface tension in 1805, and Pierre-Simon Laplace who completed the mathematical description in the following year. It is sometimes also called the Young–Laplace–Gauss equation, as Gauss unified the work of Young and Laplace in 1830, deriving both the differential equation and boundary conditions using Johann Bernoulli's virtual work principles.[2]
Contents |
Soap films
If the pressure difference is zero, as in a soap film without gravity, the interface will assume the shape of a minimal surface.
Emulsions
The equation also explains the energy required to create an emulsion. To form the small, highly curved droplets of an emulsion, extra energy is required to overcome the large pressure that results from their small radius.
Capillary pressure in a tube
In a sufficiently narrow (i.e., low Bond number) tube of circular cross-section (radius a), the interface between two fluids forms a meniscus that is a portion of the surface of a sphere with radius R. The pressure jump across this surface is:
This may be shown by writing the Young–Laplace equation in spherical form with a contact angle boundary condition and also a prescribed height boundary condition at, say, the bottom of the meniscus. The solution is a portion of a sphere, and the solution will exist only for the pressure difference shown above. This is significant because there isn't another equation or law to specify the pressure difference; existence of solution for one specific value of the pressure difference prescribes it.
The radius of the sphere will be a function only of the contact angle, θ, which in turn depends on the exact properties of the fluids and the solids in which they are in contact:
so that the pressure difference may be written as:
In order to maintain hydrostatic equilibrium, the induced capillary pressure is balanced by a change in height, h, which can be positive or negative, depending on whether the wetting angle is less than or greater than 90°. For a fluid of density ρ:
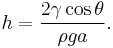 .
.
— where g is the gravitational acceleration. This is sometimes known as the Jurin rule or Jurin height[3] after James Jurin who studied the effect in 1718.[4]
For a water-filled glass tube in air at sea level:
| γ = 0.0728 J/m2 at 20 °C | θ = 20° (0.35 rad) |
| ρ = 1000 kg/m3 | g = 9.8 m/s2 |
— and so the height of the water column is given by:
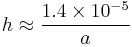 m.
m.
Thus for a 2 mm wide (1 mm radius) tube, the water would rise 14 mm. However, for a capillary tube with radius 0.1 mm, the water would rise 14 cm (about 6 inches).
Capillary action in general
In the general case, for a free surface and where there is an applied "over-pressure", Δp, at the interface in equilibrium, there is a balance between the applied pressure, the hydrostatic pressure and the effects of surface tension. The Young–Laplace equation becomes:
The equation can be non-dimensionalised in terms of its characteristic length-scale, the capillary length:
— and characteristic pressure:
For clean water at standard temperature and pressure, the capillary length is ~2 mm.
The non-dimensional equation then becomes:
Thus, the surface shape is determined by only one parameter, the over pressure of the fluid, Δp* and the scale of the surface is given by the capillary length. The solution of the equation requires an initial condition for position, and the gradient of the surface at the start point.
Axisymmetric equations
The (nondimensional) shape, r(z) of an axisymmetric surface can be found by substituting general expressions for curvature to give the hydrostatic Young–Laplace equations [5]:
Application in medicine
In medicine it is often referred to as the Law of Laplace, used in the context of cardiovascular physiology, and also respiratory physiology. Arteries may be viewed as cylinders, and the left ventricle of the heart can be viewed as part cylinder, part hemisphere (a bullet shape), modeled by the Law of Laplace as T=p x r / (2 x t), where T=wall tension, p=pressure, r=radius, t=wall thickness. For a given pressure, increased radius requires increased wall thickness to accommodate a stable wall tension; also, increased pressure requires increased thickness to maintain a stable wall tension. The latter is used to explain thickening of arteries and thickening of the left ventricle to accommodate high blood pressure. However, the thickened left ventricle is stiffer than when the thickness is normal, so it requires elevated pressures to fill, a condition known as diastolic heart failure. Note that trucks carrying pressurized gas often have multiple tubes of small radius, so that the wall tension T will be low to reduce need for thick walls to prevent pipes from bursting. The lung contains small spherical gas-exchange chambers called alveoli, where a single alveolus can be modeled as being a perfect sphere. The Law of Laplace explains why alveoli of the lung need small radius to accommodate their thin walls for gas exchange at atmospheric pressure. Numerous small radius alveoli also achieves high surface area[6]
Applying the Law of Laplace to alveoli of the lung, the pressure differential nets a force pushing on the surface of the alveolus, tending to decrease size during exhalation. The Law of Laplace states that there is an inverse relationship between surface tension and alveolar radius. It follows from this that if surface tensions are equal, a small alveolus will experience a greater inward force than a large alveolus. In that case, if both alveoli are connected to the same airway, the small alveolus will be more likely to collapse, expelling its contents into the large alveolus.
This explains why the presence of surfactant lining the alveoli is of vital importance. Surfactant reduces the surface tension on all alveoli, but its effect is greater on small alveoli than on large alveoli. Thus, surfactant compensates for the size differences between alveoli, and ensures that smaller alveoli do not collapse.[6] You can mimic this issue by connecting two inflated balloons to either ends of a plastic straw or a stiffer tube (you may need rubber bands to secure them). If the balloons are equal in thickness and radius then they can stay equally inflated, but if you squeeze one a bit to reduce its radius, the condition will be unstable, and in accord with the Law of Laplace, the smaller one will empty itself into the larger one.
The Law of Laplace also explains various phenomena encountered in the pathology of vascular or gastrointestinal walls. The wall tension in this case represents the muscular tension on the wall of the vessel. For example, if an aneurysm forms in a blood vessel wall, the radius of the vessel has increased. This means that the inward force on the vessel decreases, and therefore the aneurysm will continue to expand until it ruptures. A similar logic applies to the formation of diverticuli in the gut.[7]
The Law of Laplace can also be used to model transmural pressure in the heart and the rest of the circulatory system.
History
Francis Hauksbee performed some of the earliest observations and experiments in 1709 and these were repeated in 1718 by James Jurin who observed that the height of fluid in a capillary column was a function only of the cross-sectional area at the surface, not of any other dimensions of the column.[4][8]
Thomas Young laid the foundations of the equation in his 1804 paper An Essay on the Cohesion of Fluids [9] where he set out in descriptive terms the principles governing contact between fluids (along with many other aspects of fluid behaviour). Pierre Simon Laplace followed this up in Mécanique Céleste [10] with the formal mathematical description given above, which reproduced in symbolic terms the relationship described earlier by Young.
Laplace accepted the idea propounded by Hauksbee in the Philosophical Transactions for 1709, that the phenomenon was due to a force of attraction that was insensible at sensible distances. The part which deals with the action of a solid on a liquid and the mutual action of two liquids was not worked out thoroughly, but ultimately was completed by Gauss. Carl Neumann later filled in a few details.[8][11]
References
- ^ Surface Tension Module, by John W. M. Bush, at MIT OCW.
- ^ Robert Finn (1999). "Capillary Surface Interfaces". AMS. http://www.ams.org/notices/199907/fea-finn.pdf.
- ^ "Jurin rule". McGraw-Hill Dictionary of Scientific and Technical Terms. McGraw-Hill on Answers.com. 2003. http://www.answers.com/topic/jurin-rule?cat=technology. Retrieved 2007-09-05.
- ^ a b Jurin (1717/1719)
- ^ Lamb, H. Statics, Including Hydrostatics and the Elements of the Theory of Elasticity, 3rd ed. Cambridge, England: Cambridge University Press, 1928.
- ^ a b Sherwood, Lauralee; Justin Pearlman (2007). "Ch13". In Peter Adams. Human physiology from cells to systems (6th ed.). Thomson Brooks/Cole. ISBN 0-495-01485-0.
- ^ E. Goljan, Pathology, 2nd ed. Mosby Elsevier, Rapid Review Series.
- ^ a b [Anon.] (1911) Capillary action, Encyclopædia Britannica
- ^ Phil. Trans., 1805, p. 65
- ^ Mécanique céleste, Supplement to the tenth edition, pub. in 1806
- ^ Rouse Ball, W. W. [1908] (2003) "Pierre Simon Laplace (1749–1827)", in A Short Account of the History of Mathematics, 4th ed., Dover, ISBN 0-486-20630-0
Bibliography
- [Anon.] (1911) Capillary action, Encyclopædia Britannica
- Batchelor, G. K. (1967) An Introduction To Fluid Dynamics, Cambridge University Press
- Jurin, J. (1717/1719). "An account of some experiments shown before the Royal Society; with an enquiry into the cause of the ascent and suspension of water in capillary tubes". Philosophical Transactions of the Royal Society 30 (351–363): 739–747. doi:10.1098/rstl.1717.0026.
- Tadros T. F. (1995) Surfactants in Agrochemicals, Surfactant Science series, vol.54, Dekker