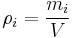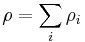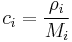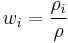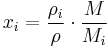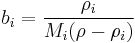Mass concentration (chemistry)
In chemistry, the mass concentration 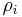 (or
(or 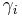 ) is defined as the mass of a constituent
) is defined as the mass of a constituent 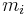 divided by the volume of the mixture
divided by the volume of the mixture 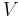 :[1]
:[1]
For a pure chemical the mass concentration equals its density.
Contents |
Definition and properties
The volume 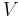 in the definition refers to the volume of the solution, not the volume of the solvent. One liter of a solution usually contains either slightly more or slightly less than 1 liter of solvent because the process of dissolution causes volume of liquid to increase or decrease. Sometimes the mass concentration is called titer.
in the definition refers to the volume of the solution, not the volume of the solvent. One liter of a solution usually contains either slightly more or slightly less than 1 liter of solvent because the process of dissolution causes volume of liquid to increase or decrease. Sometimes the mass concentration is called titer.
Dependence on volume
Mass concentration depends on the variation of the volume of the solution due mainly to thermal expansion.
Sum of mass concentration
The sum of the mass concentrations of all components (including the solvent) gives the density 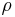 of the solution:
of the solution:
Thus, for pure component the mass concentration equals the density of the component.
Units
The SI-unit for mass concentration is kg/m3 (kilogram/cubic metre). However, more commonly the unit g/100mL is used, which is identical to g/dL (gram/decilitre).
Usage in biology
In biology, the unit "%" is sometimes incorrectly used to denote mass concentration, also called "weight/volume percentage", or "mass/volume percentage." A solution with 1 g of solute dissolved in a final volume of 100 mL of solution would be labeled as "1 %" or "1 % w/v" (weight/volume). The notation is mathematically flawed because the unit "%" can only be used for dimensionless quantities. "Percent solution" or "percentage solution" are thus terms best reserved for "weight percent solutions" (w/w = wt% = weight solute/weight total solution after mixing), or "volume percent solutions" (v/v = v% = volume solute per volume of total solution after mixing). The very ambiguous terms "percent solution" and "percentage solutions" with no other qualifiers, continue to occasionally be encountered.
This common usage of % to mean wt/v in biology is because of many biological solutions being dilute and water-based or an aqueous solution. Liquid water has a density of approximately 1 g/cm3 (1 g/ml) (water density). Thus 100 ml of water is equal to approximately 100 g. Therefore, a solution with 1 g of solute dissolved in final volume of 100 ml aqueous solution may also be considered 1 % w/w (1 g solute in 100 g water). This approximation breaks down as the solute concentration is increased. For an example, refer to the densities of water-NaCl mixtures (density of water with dissolved NaCl). High solute concentrations are often not physiologically relevant, but are occasionally encountered in pharmacology, where the weight per volume notation is still sometimes encountered. An extreme example is saturated solution of potassium iodide (SSKI) which attains 100 "%" wt/v potassium iodide mass concentration (1 gram KI per mL solution) only because the solubility of the dense salt KI is extremely high in water, and the resulting solution is very dense (1.72 times as dense as water).
Although there are examples to the contrary, it should be stressed that the commonly used "units" of % w/v are grams/milliliters (g/ml). 1% w/v solutions are sometimes thought of as being gram/100 ml but this detracts from the fact that % w/v is g/ml; 1 g of water has a volume of approximately 1 ml (at STP) and the mass concentration is said to be 100%. To make 10 ml of an aqueous 1% cholate solution, 0.1 grams of cholate are dissolved in 10 ml of water. Volumetric flasks are the most appropriate piece of glassware for this procedure as deviations from ideal solution behavior can occur with high solute concentrations.
In solutions, mass concentration is commonly encountered as the ratio of weight/[volume solution], or wt/volume. In water solutions containing relatively small quantities of dissolved solute (as in biology), such figures may be "percentivized" by multiplying by 100 a ratio of grams solute per mL solution. The result is given as "weight/volume percentage," or "mass/volume percentage." Such a convention expresses mass concentration of 1 gram of solute in 100 mL of solution, as "1 w/v %."
Related Quantities
Molar concentration
The conversion to molar concentration 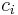 is given by:
is given by:
where 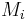 is the molar mass of constituent
is the molar mass of constituent 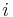 .
.
Mass fraction
The conversion to mass fraction 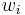 is given by:
is given by:
Mole fraction
The conversion to mole fraction 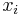 is given by:
is given by:
where 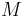 is the average molar mass of the mixture.
is the average molar mass of the mixture.
Molality
For binary mixtures, the conversion to molality 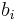 is given by:
is given by:
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "mass concentration".
|
||||||||||||||