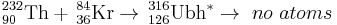Unbihexium
|
||||||
| Appearance | ||||||
|---|---|---|---|---|---|---|
| General properties | ||||||
| Name, symbol, number | unbihexium, Ubh, 126 | |||||
| Pronunciation | /ˌuːnbaɪˈhɛksiəm/ | |||||
| Element category | superactinide | |||||
| Group, period, block | n/a, 8, g | |||||
| Standard atomic weight | unknown | |||||
| Electron configuration | [Uuo] 5g6 8s2 (predicted) |
|||||
| Electrons per shell | 2, 8, 18, 32, 38, 18, 8, 2 (predicted) (Image) |
|||||
| Physical properties | ||||||
| Color | unknown | |||||
| Phase | Unknown (expected to be a solid) | |||||
| Atomic properties | ||||||
Unbihexium ( /ˌuːnbaɪˈhɛksiəm/), also known as eka-plutonium or element 126, is a hypothetical chemical element with atomic number 126 and symbol Ubh. It is of interest because of its location at the peak of the hypothesized island of stability.
Contents |
History
The first attempt to synthesize unbihexium was performed in 1971 by Bimbot et al. using the hot fusion reaction:
A high energy alpha particle was observed and taken as possible evidence for the synthesis of unbihexium. Recent research suggests that this is highly unlikely as the sensitivity of experiments performed in 1971 would have been several orders of magnitude too low according to current understanding.
To date, no other attempt has been made to synthesize unbihexium.
Target-projectile combinations leading to Z=126 compound nuclei
The table below contains various combinations of targets and projectiles (both at max no. of neutrons) which could be used to form compound nuclei with Z=126. The only practical isotopes of unbihexium that would be considerably longer-lived than others are 310Ubh and 322Ubh.
| Target | Projectile | CN | Attempt result |
|---|---|---|---|
| 182Hf | 136Xe | 318Ubh | Reaction yet to be attempted |
| 232Th | 84Kr | 316Ubh | Failure to date |
| 243Cm | 67Zn | 310Ubh | Reaction yet to be attempted |
| 248Cf | 62Ni | 310Ubh | Reaction yet to be attempted |
| 249Cf | 61Ni | 310Ubh | Reaction yet to be attempted |
| 251Es | 59Co | 310Ubh | Reaction yet to be attempted |
| 254Fm | 56Fe | 310Ubh | Reaction yet to be attempted |
| 257Md | 53Mn | 310Ubh | Reaction yet to be attempted |
| 260Rf | 50Ti | 310Ubh | Reaction yet to be attempted |
| 271Sg | 48Ca | 319Ubh | Reaction yet to be attempted |
Another way to synthesize unbihexium would be to overshoot it by fusion of 130Te and 204Hg; successive alpha decay of the compound nucleus 334Utb would land right on 322Ubh (predicted to be relatively stable). 130Te constitutes about 34% of the natural element; however, 204Hg only constitutes about 7% of natural mercury.
Stable unbihexium
Calculations according to the Hartree-Fock-Bogoliubov Method using the non-relativistic Skyrme interaction have proposed Z=126 as a closed proton shell. In this region of the periodic table, N=184 and N=196 have been suggested as closed neutron shells. Therefore the isotopes of most interest are 310Ubh and 322Ubh, for these might be considerably longer-lived than other isotopes.
Predicted chemistry
Unbihexium is predicted to belong to a new block of valence g-electron atoms, although the g-block's position left of the f-block is speculative. The expected electron configuration is [Uuo]5g6 8s2 although there may be a smearing out of the energies of 5g, 6f and 7d orbitals.
Recent calculations have suggested a stable monofluoride UbhF may exist, resulting from a bonding interaction between the 5g orbital on Ubh and the 2p orbital on fluorine.[1] Other predicted oxidation states include III, IV, VI, and VIII.
See also
References
- ^ Jacoby, Mitch (2006). "As-yet-unsynthesized superheavy atom should form a stable diatomic molecule with fluorine". Chemical & Engineering News 84 (10): 19. http://pubs.acs.org/cen/news/84/i10/8410notw9.html. Retrieved 2008-01-14.
| H | He | |||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | |||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | |||||||||||
| Uue | Ubn | Ute | Uqn | Uqu | Uqb | Uqt | Uqq | Uqp | Uqh | Uqs | Uqo | Uqe | Upn | Upu | Upb | Upt | Upq | Upp | Uph | Ups | Upo | Upe | Uhn | Uhu | Uhb | Uht | Uhq | Uhp | Uhh | Uhs | Uho | |||||||||||
| Uhe | Usn | |||||||||||||||||||||||||||||||||||||||||
| Ubu | Ubb | Ubt | Ubq | Ubp | Ubh | Ubs | Ubo | Ube | Utn | Utu | Utb | Utt | Utq | Utp | Uth | Uts | Uto | |||||||||||||||||||||||||
| Usu | Usb | Ust | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||