Polyphosphate
Triphosphates are salts or esters of polymeric oxyanions formed from tetrahedral PO4 (phosphate) structural units linked together by sharing oxygen atoms. When two corners are shared the polyphosphate may have a linear chain structure or a cyclic ring structure. In biology the polyphosphate esters AMP, ADP and ATP are involved in energy transfer. A variety of polyphosphates find application in mineral sequestration in municipal waters, generally being present at 1 to 5 pm.[1] GTP, CTP, and UTP are also nucleotides important in the protein synthesis, lipid synthesis and carbohydrate metabolism, respectively.
Contents |
Structure and formation
The structure of tripolyphosphoric acid illustrates the principles which define the structures of polyphosphates. It consists of three tetrahedral PO4 units linked together by sharing oxygen atoms. Structurally, the outer tetrahedra share one vertex with the central tetrahedron; the central tetrahedron shares two corners with the others tetrahedra. The corresponding phosphates are related to the acids by loss of the acidic protons. In the case of the cyclic trimer each tetrahedron shares two vertices with adjacent tetrahedra.
Sharing of three corners is possible as in the sheet-structure Phyllosilicates, but such structures occur only under extreme conditions. Three-corner sharing also occurs in phosphorus pentoxide, P4O10, which has a 3-dimensional structure.
Chemically, the polymerization reaction can be seen as a condensation reaction. The process begins with two phosphate units coming together.
- 2 PO43− + 2 H+ P2O74− + H2O
It is shown as an equilibrium reaction because it can go in the reverse direction, when it is known as an hydrolysis reaction because a water molecule is split (Lysed). The process may continue in steps; at each step another PO3 unit is added to the chain, as indicated by the part in brackets in the illustration of polyphosphoric acid. P3O10 can be seen as the end product of condensation reactions, where each tetrahedron shares three cornes with the others. Conversely, a complex mix of polymers is produces when a small amount of water is added to phosphorus pentoxide.
Acid-base and complexation properties
Polyphosphates are weak bases. A lone pair of electrons on an oxygen atom can be donated to a hydrogen ion (proton) or a metal ion in a typical Lewis acid-Lewis base interaction. This has profound significance in biology. For instance, adenosine triphosphate is about 25% protonated in aqueous solution at pH 7.[2]
- ATP4- + H+ ATPH3-, pKa
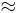 6.6
6.6
Further protonation occurs at lower pH values.
ATP forms chelate complexes with metal ions. The stability constant for the equilibrium
- ATP4- + Mg2+ MgATP2-, log β
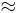 4
4
is particularly large.[3] The formation of the magnesium complex is a critical element in the process of ATP hydrolysis, as it weakens the link between the terminal phosphate group and the rest of the molecule.[2][4]
The "high energy" phosphate bond
The energy released in ATP hydrolysis,
- ATP4- + H2O → ADP3- +Pi-
at ΔG 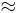 -36.8 kJ mol−1 is large by biological standards. Pi stands for inorganic phosphate, which is protonated at biological pH. However, it is not large by inorganic standards. The term "high energy" refers to the fact that it is high relative to the amount of energy released in the organic chemical reactions that can occur in living systems.
-36.8 kJ mol−1 is large by biological standards. Pi stands for inorganic phosphate, which is protonated at biological pH. However, it is not large by inorganic standards. The term "high energy" refers to the fact that it is high relative to the amount of energy released in the organic chemical reactions that can occur in living systems.
High-polymeric inorganic polyphosphates
High-polymeric inorganic polyphosphates were found in living organisms by L. Liberman in 1890. These compounds are linear polymers containing a few to several hundred residues of orthophosphate linked by energy-rich phosphoanhydride bonds.
Previously, it was considered either as “molecular fossil” or as only a phosphorus and energy source providing the survival of microorganisms under extreme conditions. These compounds now known to also have regulatory roles and to occur in representatives of all kingdoms of living organisms, participating in metabolic correction and control on both genetic and enzymatic levels. Polyphosphate is directly involved in the switching-over of the genetic program characteristic of the exponential growth stage of bacteria to the program of cell survival under stationary conditions, “a life in the slow line”. They participate in many regulatory mechanisms occurring in bacteria:
- They participate in the induction of rpoS, an RNA-polymerase subunit which is responsible for the expression of a large group of genes involved in adjustments to the stationary growth phase and many stressful agents.
- They are important for cell motility, biofilms formation and virulence.
- Polyphosphates and exopolyphosphatases participate in the regulation of the levels of the stringent response factor, guanosine 5'-diphosphate 3'-diphosphate (ppGpp), a second messenger in bacterial cells.
- Polyphosphates participate in the formation of channels across the living cell membranes. The above channels formed by polyphosphate and poly-b-hydroxybutyrate with Ca2+ are involved in the transport processes in a variety of organisms.
- An important function of polyphosphate in microorganisms—prokaryotes and the lower eukaryotes—is to handle changing environmental conditions by providing phosphate and energy reserves. Polyphosphates are present in animal cells, and there are many data on its participation in the regulatory processes during development and cellular proliferation and differentiation—especially in bone tissues and brain.
In humans polyphosphates are shown to play a key role in blood coagulation. Produced and released by platelets they activate Factor XII which is essential for blood clot formation. Furthermore platelets-derived polyphosphates activate blood coagulation factor XII (Hageman factor) that initiates fibrin formation and the generation of a proinflammatory mediator, bradykinin that contributes to leakage from the blood vessels and thrombosis.[5][6]
See also
References
- ^ http://www.jacksmagic.com/pdfs/FAQ_phosphates.pdf
- ^ a b Storer A, Cornish-Bowden A (1976). "Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions". Biochem J 159 (1): 1–5. PMC 1164030. PMID 11772. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1164030.
- ^ Wilson J, Chin A (1991). "Chelation of divalent cations by ATP, studied by titration calorimetry". Anal Biochem 193 (1): 16–9. doi:10.1016/0003-2697(91)90036-S. PMID 1645933.
- ^ Garfinkel L, Altschuld R, Garfinkel D (1986). "Magnesium in cardiac energy metabolism". J Mol Cell Cardiol 18 (10): 1003–13. doi:10.1016/S0022-2828(86)80289-9. PMID 3537318.
- ^ Müller F, Mutch, NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T (Dec 2009). "Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo". CELL 139 (6): 1143–56. doi:10.1016/j.cell.2009.11.001. PMC 2796262. PMID 20005807. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2796262.
- ^ "Newly discovered mechanism by which blood clots form". physorg.com. December 10, 2009. http://www.physorg.com/news179673245.html. Retrieved 13 December 2009.
External links
- Pavlov E, Grimbly C, Diao CT, French RJ (September 2005). "A high-conductance mode of a poly-3-hydroxybutyrate/calcium/polyphosphate channel isolated from competent Escherichia coli cells". FEBS Lett. 579 (23): 5187–92. doi:10.1016/j.febslet.2005.08.032. PMID 16150446. http://linkinghub.elsevier.com/retrieve/pii/S0014-5793(05)01027-6.
- Kulaev I, Vagabov V, Kulakovskaya T (1999). "New aspects of inorganic polyphosphate metabolism and function". J. Biosci. Bioeng. 88 (2): 111–29. doi:10.1016/S1389-1723(99)80189-3. PMID 16232585. http://linkinghub.elsevier.com/retrieve/pii/S1389-1723(99)80189-3.
- Kulaev I, Kulakovskaya T (2000). "Polyphosphate and phosphate pump". Annu. Rev. Microbiol. 54: 709–34. doi:10.1146/annurev.micro.54.1.709. PMID 11018142. http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.micro.54.1.709.