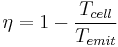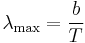Thermophotovoltaic
Thermophotovoltaic (TPV) energy conversion is a direct conversion process from heat differentials to electricity via photons. A basic thermophotovoltaic system consists of a thermal emitter and a photovoltaic diode cell.
The temperature of the thermal emitter varies between different systems from about 900 °C to about 1300 °C, although in principle TPV devices can extract energy from any emitter with temperature elevated above that of the photovoltaic device (forming an optical heat engine). The emitter can be a piece of solid material or a specially engineered structure. A conventional solar cell is effectively a TPV device in which the Sun functions as the emitter. Thermal emission is the spontaneous emission of photons due to thermal motion of charges in the material. For normal TPV temperatures, this radiation is mostly at near infrared and infrared frequencies. The photovoltaic diodes can absorb some of these radiated photons and convert them into free charge carriers, that is electricity.
Thermophotovoltaic systems have few, if any, moving parts and are therefore very quiet and require low maintenance. These properties make thermophotovoltaic systems suitable for remote-site and portable electricity-generating applications. Their efficiency-cost properties, however, are often rather poor compared to other electricity-generating technologies. Current research in the area aims at increasing the system efficiencies while keeping the system cost low.
In the design of a TPV system, it is usually desired to match the optical properties of thermal emission (wavelength, polarization, direction) with the most efficient conversion characteristics of the photovoltaic cell, since unconverted thermal emission is a major source of inefficiency. Most groups focus on gallium antimonide (GaSb) cells. Germanium (Ge) is also suitable.[1] Much research and development in TPVs therefore concerns methods for controlling the emitter's properties.
TPV cells have often been proposed as auxiliary power conversion devices for regeneration of lost heat in other power generation systems, such as steam turbine systems or solar cells.
A prototype TPV hybrid car was even built. The "Viking 29" [2] (TPV) powered automobile, designed and built by the Vehicle Research Institute (VRI) at Western Washington University.
TPV research is a very active area. Among others, the University of Houston TPV Radioisotope Power Conversion Technology development effort is aiming at combining thermophotovoltaic cell concurrently with thermocouples to provide a 3 to 4-fold improvement in system efficiency over current radioisotope thermoelectric generators.
Contents |
History
Though Henry Kolm had constructed an elementary TPV system at MIT in 1956, Pierre Aigrain is widely cited as the inventor of TPV based on the content of some lectures he gave at MIT between 1960–1961 which, unlike Kolm's system, led to research and development.[3] A review of the historical development of TPV is presented in Nelson (2003).[3]
Background
Thermophotovoltaics (TPVs) are a class of power generating systems that are used to convert thermal energy into electrical energy. They consist of, at a minimum, an emitter and a photovoltaic power converter. However, most TPV systems also include additional components such as concentrators, filters and reflectors. The basic principle of operation is similar to that of traditional photovoltaics (PV) where a p-n junction is used to absorb optical energy, generate and separate electron/hole pairs, and in doing so convert that energy into electrical power. The difference is that the optical energy is not directly generated by the Sun, but instead by a material at high temperature (termed the emitter), causing it to emit light. In this way thermal energy is converted to electrical energy.
The emitter can be heated by sunlight or combustion. In this sense, TPVs provide a great deal of versatility in potential fuels. In the case of solar TPVs, extremely large concentrators are needed to provide reasonable temperatures for efficient operation.
Vast improvements can be made on this basic concept by taking advantage of filters or selective emitters to create emissions in a narrow wavelength range that is optimized for the specific photovoltaic (PV) converter used in the system. In this way TPVs can overcome a fundamental challenge for traditional PVs, making efficient use of the entire solar spectrum. For blackbody emitters, photons with energy less than the bandgap of the converter cannot be absorbed to generate electron-hole pairs, and are either reflected and lost or pass through the cell. Photons with energy above the bandgap can be absorbed, but the excess energy, 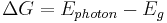 , is again lost, generating undesirable heating in the cell. In the case of TPVs, similar issues can exist, but the use of either selective emitters (emissivity over only a narrow wavelength range), or optical filters that only pass a narrow range of wavelengths and reflect all others, can be used to generate emission spectra that can be optimally converted by the PV device.
, is again lost, generating undesirable heating in the cell. In the case of TPVs, similar issues can exist, but the use of either selective emitters (emissivity over only a narrow wavelength range), or optical filters that only pass a narrow range of wavelengths and reflect all others, can be used to generate emission spectra that can be optimally converted by the PV device.
To achieve the maximum efficiency, all photons should be converted. A process often termed photon recycling can be used to approach this. Here reflectors are placed behind the converter and anywhere else in the system that photons might not be efficiently directed to the collector. These photons are directed back to the concentrator where they can be converted, or back to the emitter, where they can be reabsorbed to generate heat and additional photons. An idealized TPV system would use photon recycling and selective emission to convert all photons into electricity.
Efficiency
To understand the overall benefit of TPV systems, a discussion of the basic principles of efficiency in TPVs is useful. The absolute upper limit for efficiency in TPVs (and all systems that convert heat energy to work) is the Carnot efficiency, that of an ideal heat engine. This efficiency is given by:
where Tcell is the temperature of the PV converter. For the best reasonable values in a practical system, Tcell~300K and Temit~1800, giving a maximum efficiency of ~83%. This limit sets the upper limit for the system efficiency. At 83% efficiency, all heat energy is converted to radiation by the emitter which is then converted by the PV into electrical energy without losses, such as thermalization or Joule heating. At the maximum efficiency, we also assume that there is no entropy change, which is only possible if the emitter and cell are at the same temperature. Still, as an upper limit, it is useful. Because of the complexity of TPV systems and the many sources of inefficiency, more accurate models for efficiency become quite complicated, but a discussion of the various sources of inefficiency that cause real systems to fall far short of this limit is worthwhile.
Emitters
For the emitter, deviations from perfect absorbing and perfect blackbody behavior lead to light losses. For the case of selective emitters, any light emitted at wavelengths not matched to the bandgap energy of the PV may not be efficiently converted (for reasons discussed above) and leads to reduced efficiency. In particular, emissions associated with phonon resonances are difficult to avoid for wavelengths of in the deep IR, which cannot be practically converted. Ideally, an emitter will not emit in this range, and energy will only be converted at wavelengths that are easily converted.
Filters
For blackbody emitters or imperfect selective emitters, filters are needed to reflect non-ideal wavelengths back to the emitter. In practice, these filters are rarely perfect. Any light that is absorbed or scattered and not redirected to the emitter or the converter is lost. Additionally, practical filters often reflect a small percentage of light in desired wavelength ranges or transmit light of non-ideal wavelengths. Both can lead to inefficiencies.
Converters
Even for systems where only light of optimal wavelengths is passed to the converter, inefficiencies associated with non-radiative recombination and ohmic losses exist. Since these losses can depend on the intensity of light incident on the cell, real systems must consider the intensity produced by a given set of conditions (emitter material, filter, operating temperature).
Geometry
In an ideal system, the emitter would be surrounded by PV converters so no light is lost. However, realistically, geometries must accommodate the input energy (fuel injection or input light) used to heat the emitter. Additionally, high costs prohibit the placement of converters everywhere. When the emitter reemits light, anything that does not travel to the converters is lost. Mirrors can be used to redirect some of this light back to the emitter; however, the mirrors may have their own losses.
Blackbody radiation
To understand some of the practical demands of real TPV components, looking at some basic numbers is useful. For the purposes of these arguments we will discuss blackbody emitters where photon recirculation is achieved via filters; however, similar concepts can be applied towards selective emission emitters. Planck's law states that a blackbody will emit light with a spectrum given by:
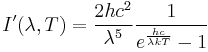
where I' is the flux of light of a specific wavelength, λ, given in units of 1/m3/s. Here, h is Planck constant, k is Boltzmann constant, c is the speed of light, and Temit is the temperature of the emitter. Thus, the flux of light with wavelengths in a specific range can be found by integrating over the range. The peak wavelength is determined by the temperature, Temit based on Wien’s displacement law:
where b is Wien’s displacement constant. For most materials, the maximum temperature an emitter can stably operate at is about 1800 °C. This corresponds to an intensity which is peaked at λ~1600 nm or an energy of ~0.75 eV. For more reasonable operation temperatures of 1200 °C, this drops to ~0.5 eV. These energies dictate the range of bandgaps that are needed for practical TPV converters (though the peak spectral power is slightly higher). Traditional PV materials such as Si (1.1 eV) and GaAs (1.4 eV) are substantially less practical for TPV systems, as the intensity of the blackbody spectrum is extremely low at these energies for emitters at realistic temperatures.
Active components and materials selection
Emitters
Efficiency, temperature resistance, and cost are the three major factors when choosing the radiator for TPVs. Efficiency is determined by energy absorbed relative to total incoming radiation. Ability to operate at high temperatures is a crucial factor because efficiency increases with operating temperature. As emitter temperature increases, the blackbody radiation shifts to shorter wavelengths, allowing for more efficient absorption by photovoltaic cells. Lastly, cost is a major limitation in commercialization of TPVs.
Polycrystalline silicon carbide
Polycrystalline silicon carbide (SiC) is the most commonly used emitter for burner TPVs. SiC is thermally stable to ~1700 °C. However, SiC radiates much of its energy in the long wavelength regime, far lower in energy than even the narrowest bandgap photovoltaic. This radiation, in turn, is not converted into electrical energy. However, non-absorbing selective filters in front of the PV,[4] or mirrors deposited on the back side of the PV[5] can be used to reflect the long wavelengths back to the emitter, thereby recycling the unconverted energy. In addition, polycrystalline SiC is extremely cheap to manufacture, making it a good choice for commercial applications.
Tungsten
Refractory metals are often used as selective emitters for burner TPVs with tungsten being the most common choice. Tungsten has higher emissivity in the visible and near-IR range of 0.45 to 0.47 and a low emissivity of 0.1 to 0.2 in the IR region.[6] The emitter is usually in the shape of a cylinder with a sealed bottom, which can be considered a cavity. The emitter is attached to the back of a thermal absorber such as SiC and maintains the same temperature. Emission occurs in the visible and near IR range which can be readily converted by the PV to electrical energy.
Rare-earth oxides
Rare-earth oxides such as ytterbium oxide (Yb2O3) and erbium oxide (Er2O3) are the most commonly used selective emitters for TPVs. These oxides emit a narrow band of wavelengths in the near-infrared region, allowing the tailoring of the emission spectra to better fit the absorbance characteristics of a particular PV cell. The peak of the emission spectrum occurs at 1.29 eV for Yb2O3 and 0.827 eV for Er2O3. As a result, Yb2O3 can be used a selective emitter for Si PV cells and Er2O3, for GaSb or InGaAs. However, the slight mismatch between the emission peaks and band gap of the absorber results in a significant loss of efficiency. In addition, selective emission only becomes significant at 1100 °C and increases with temperature, per Planck’s Law. At reasonable operating temperatures (below 1700 °C), selective emission of rare-earth oxides is fairly low, resulting in a further decrease in efficiency. Currently, only 13% efficiency has been achieved with Yb2O3 and silicon PV cells. In general selective emitters have had limited success. More often filters are used with blackbody emitters to pass wavelengths matched to the bandgap of the PV and reflect mismatched wavelengths back to the emitter.
Photonic crystals
Photonic crystals are a class of novel periodic materials that allow the precise control of electromagnetic wave properties. These materials give rise to the photonic bandgap (PBG). In the spectral range of the PBG, electromagnetic waves cannot propagate. The engineering of these materials allows some ability to tailor their emission and absorption properties, allowing for more effective design of selective emitters. Selective emitters with peaks at higher energy than the blackbody peak (for practical TPV temperatures) allow for wider bandgap converters. These converters are traditionally cheaper to manufacture and less temperature sensitive. Recently, researchers at Sandia Labs have demonstrated a high-efficiency (34% of light emitted from PBG selective emitter was converted to electricity) TPV system using tungsten photonic crystals.[7] However, manufacturing of these devices is difficult and not currently commercially feasible.
Photovoltaic cells
Silicon
Early work in TPVs focused on the use of Si PVs. Silicon’s commercial availability, extremely low cost, scalability, and ease of manufacture makes this material an extremely appealing candidate. However, the relative wide bandgap of Si (1.1eV) is not ideal for use with a blackbody emitter at lower operating temperatures. Calculations using Planck’s law, which describes the blackbody spectrum as a function of temperature, indicates that Si PVs would only be feasible at temperatures much higher than 2000 K. No emitter has been demonstrated that can operate at these temperatures. These engineering difficulties led to the pursuit of lower-bandgap semiconductor PVs for conversion of the blackbody spectrum.
However, using selective radiators with Si PVs is still a possibility. Selective radiators would eliminate high and low energy photons, reducing heat generated. Ideally, selective radiators would emit no radiation above and below the band edge of the PV converter, increasing conversion efficiency significantly. However, selective emitters today are far from ideal. Consequently, no efficient TPVs have been realized using a Si PVs.
Germanium
Early investigations into low bandgap semiconductors focused on germanium (Ge). Ge has a bandgap of 0.66 eV, allowing for conversion of a much higher fraction of incoming radiation. However, poor performance was observed due the extremely high effective electron mass of Ge. Compared to III-V semiconductors, Ge’s high electron effective mass leads to a high density of states in the conduction band and therefore a high intrinsic carrier concentration. As a result, Ge diodes have fast decaying “dark” current and therefore, a low open-circuit voltage. In addition, surface passivation of germanium has proven extremely difficult. These reasons make germanium an unlikely candidate for use in TPVs.
Gallium antimonide
The gallium antimonide (GaSb) PV cell, invented in 1989,[8] is the basis of most PV cells in modern TPV systems. GaSb is a III-V semiconductor with the zinc blende crystal structure. The GaSb cell is recognized a key development in the TPV community owing to its narrow bandgap of 0.72 eV. This allows GaSb to respond to light at longer wavelengths than the conventional silicon solar cell thus enabling higher power densities when used in conjunction with manmade emission sources. A solar cell with 35% efficiency was demonstrated by the inventors at Boeing in 1989 using a bilayer PV with GaAs and GaSb,[8] setting the world record for solar cell efficiency.
The manufacturing process for the GaSb PV cell is quite simple. Czochralski Te-doped n-type GaSb wafers are readily commercially available. Vapor-based Zn diffusion is then carried out at elevated temperatures ~450 °C to allow for p-type doping. Lastly, front and back electrical contacts are patterned using traditional photolithography techniques and an anti-reflective coating is deposited. Current efficiencies are estimated at ~20% using a 1000 °C blackbody spectrum.[9] The radiative limit for efficiency of the GaSb cell in this setup is 52%, so vast improvements can still be made.
Indium gallium arsenide antimonide
Indium gallium arsenide antimonide (InGaAsSb) is a compound III-V semiconductor. The addition of GaAs allows for a narrower bandgap (0.5 to 0.6 eV), and therefore better absorption of long wavelengths. Specifically, the bandgap has been engineered to 0.55 eV. With this bandgap, the compounded achieved a photon-weighted internal quantum efficiency of 79% with a fill factor of 65% for a blackbody at 1100 °C.[10] This was for a device grown on a GaSb substrate by organometallic vapor phase epitaxy (OMVPE). Devices have also been grown by molecular beam epitaxy (MBE) and liquid phase epitaxy (LPE). The internal quantum efficiencies (IQE) of these devices have all been impressive. The IQE of the LPE-grown devices are approaching 90% while devices grown by the other two techniques exceed 95%.[11] The largest problem with InGaAsSb cells is phase separation. Compositional inconsistencies throughout the device and are extremely detrimental to its performance. When phase separation can be avoided, the IQE and fill factor of InGaAsSb are approaching theoretical limits in wavelength ranges near the bandgap energy, however, the Voc/Eg ratio is far from the ideal.[11] Improving this ratio through photon recycling and tandem cell structures would be the next area in which the performance of this material could be significantly improved. In addition, current methods to manufacture InGaAsSb PVs are expensive and not commercially viable.
Indium gallium arsenide
Indium gallium arsenide (InGaAs) is also a compound III-V semiconductor. It can be applied in two ways for use in TPVs. When lattice-matched to an InP substrate, InGaAs has a bandgap of 0.74 eV, which is not an improvement on traditional GaSb. Devices of this configuration have been produced with a fill factor of 69% and an efficiency of 15%.[12] However, to absorb higher wavelength photons, the bandgap may be engineered by changing the ratio of In to Ga. The range of bandgaps for this system is from about 0.4 to 1.4 eV. However, these different structures cause strain with the InP substrate. This can be controlled with graded layers of InGaAs with different compositions. This was done to develop of device with a quantum efficiency of 68% and a fill factor of 68% grown by molecular beam epitaxy.[10] This device also had a bandgap of 0.55 eV achieved in the compound In0.68Ga0.33As. InGaAs has the advantage of being a well-developed material. InGaAs can also be made to lattice match perfectly with Ge resulting in very low defect densities. Being able to use Ge as a substrate is a significant advantage over more expensive or harder to produce substrates.
Indium phosphide arsenide antimonide
The InPAsSb quaternary alloy has been grown by both OMVPE and LPE. When lattice-matched to InAs, it has a bandgap in the range 0.3–0.55 eV. The benefits of a TPV system with such a low band gap have not been studied significantly. Therefore, cells incorporating InPAsSb have not been optimized and do not yet have very competitive properties and performance. The longest spectral response from an InPAsSb cell studied was out to 4.3 μm with a maximum response at 3 μm.[11] While this is a promising material in the very low bandgap range, it has yet to be developed. For this and other extremely low-bandgap materials, high IQE for long wavelengths is hard to achieve due to an increase in Auger recombination.
Applications of thermophotovoltaics
TPVs have significant promise for efficient and economically viable power systems for both military and commercial applications. Compared to traditional nonrenewable energy sources, burner TPVs have little NOx emissions and are virtually silent. Solar TPVs, on the other hand, are a source of entirely renewable energy with no emissions. Compared to photovoltaics, TPVs can be more efficient owing to recycling of unabsorbed photons. However, the structure of TPVs is more complex, and losses at each energy conversion step can result in a lower efficiency than that of photovoltaics. Further developments must be made to the absorber/emitter and PV cell to realize its full potential as a renewable energy source. Unlike PVs, however, when TPVs are used with a burner source, they provide on-demand energy. As a result, no form of energy storage is needed. In addition, owing to the PV’s proximity to the radiative source, TPVs can generate current densities 300 times that of conventional PVs.
Government applications
Man-portable power
With the increased usage of electronics on the battlefield, there is a need to provide portable power sources to soldiers. Conventional diesel generators are far too heavy for personal use in the field. Scalability allows TPVs to be smaller and lighter than conventional generators. In addition TPVs have very little emission and are silent, making it feasible for tactical field application. Multifuel operation is another potential future benefit.
Early investigations into TPVs in the 1970s proved to be impossible due to PV limitations. However, with the realization of the GaSb photocell, a renewed effort in the 1990s produced greater results. In early 2001, JX Crystals delivered a TPV based battery charger to the Army that produced an output of 230 W by burning propane. This prototype utilized SiC emitter operating at 1250 °C and GaSb photocells and was approximately 0.5 m tall.[13] The power source had an efficiency of 2.5%, calculated by the ratio of the power generated to the thermal energy of the fuel burned. This is too low for practical use on the battlefield. To increase efficiency, narrow-band emitters would need to be realized and the temperature of the burner would need to be raised. To accommodate this, further thermal management steps, such as water cooling or coolant boiling, must be implemented. Although many successful proof-of-concept prototypes have been demonstrated, no portable TPV power sources have been developed for troop testing or battlefield implementation.
Spacecraft
For space travel power generation systems are needed that provide consistent and reliable power without requiring storage of large amounts of fuel. As a result, solar and radioisotope fuels (extremely high power density and long lifetime) are ideal sources of energy. TPVs have been proposed as sources for conversion for each. In the case of solar energy, orbital spacecraft may be better locations for the large and potentially cumbersome concentrators required for practical TPVs. However, because of weight considerations and inefficiencies associated with the somewhat more complicated design of TPVs, conventional PVs will almost surely be more effective for these applications. If the efficiency of individual components can be improved to the point that TPVs can offer substantially higher conversion efficiencies than PVs owing to photon recycling, then they might become useful for solar conversion in space.
Probably more interesting is the prospect of using TPVs for conversion of radioisotope energy. The output of isotopes is already thermal energy, so in this sense TPVs are optimal. In the past thermoelectricity, which is also direct thermal to electrical conversion with no moving parts, has been used because of the extremely low TPV efficiencies compared to the ~10% of thermoelectric converters.[14] Stirling engines have also been considered, but are undesirable due to reliability concerns, which are unacceptable for space missions, despite improved conversion efficiencies (>20%).[15] However, with the recent advances in small-bandgap PVs critical for effective operation, TPVs are becoming more promising candidates. For example, a TPV radioisotope converter with a 20% efficiency was demonstrated that uses a tungsten emitter heated to 1350 K, with tandem filters and a 0.6 eV bandgap InGaAs PV converter (cooled to room temperature). About 30% of the lost energy was due to the optical cavity and filters. The remainder was due to the efficiency of the PV converter.[15]
Low-temperature operation of the converter is critical to the efficiency of TPV. Heating PV converters increases their dark current, thereby reducing the overall efficiency. For all TPV systems, the converter will be heated by the radiation from the emitter. In terrestrial systems it is reasonable to dissipate this heat without using additional energy by heat sinking the converter. However, space is an isolated system, and such heat sinks are not practical. Therefore, it is critical to develop innovative solutions to efficiently remove that heat, or optimized TPV cells that can operate efficiently with higher temperature converters. Both represent substantial challenges. Despite this, TPVs offer substantial promise for use in future space travel.[14]
Commercial applications
Off-grid generators
Many homes in North America as well as developing countries are located in remote regions not connected to the power grid. Where available, power line extensions can be extremely expensive and impractical. TPVs can provide a continuous supply of power in off-grid homes. Traditional PVs on the other hand, would not provide sufficient power during the winter months and nighttime, while TPVs can utilize alternative fuels to augment solar-only production
The greatest advantage for TPV generators is cogeneration of heat and power. In cold climates, it can function as both a heater or stove and a power generator. JX Crystals has developed a prototype TPV heating stove and generator. It burns natural gas and uses a SiC source emitter operating at 1250 °C and GaSb photocell to output 25,000 BTU/hr simultaneously generating 100 W. However, costs must be significantly reduced to render it commercially viable.
When a furnace is used as a heater and a generator, it is called combined heat and power (CHP). Many TPV CHP scenarios have been theorized but a generator using boiling coolant was found most cost efficient.[16] The proposed CHP would utilize a SiC IR emitter operating at 1425 °C and GaSb photocells cooled by boiling coolant. The TPV CHP would output 85,000 BTU/hr and generate 1.5 kW. The estimated efficiency would be 12.3% and the investment would be 0.08 €/kWh provided that the lifetime of the CHP furnace is 20 years. The estimated cost of other non-TPV CHPs are 0.12 €/kWh for gas engine CHP and 0.16 €/kWh for fuel cell CHP. This proposed furnace has not been developed because there is comparatively a very small market for off-grid power generation and no funding is available to develop a GaSb PV array cooled by boiling liquid.
Recreational vehicles
TPVs have been proposed for use in recreational vehicles. With the advent of hybrid and other electrically powered vehicles, power generators with electrical outputs have become more interesting. In particular the versatility of TPVs for fuel choice and the ability to use multiple fuel sources makes them interesting as a wider variety of fuels are being with better sustainability are being investigated today. Furthermore, the silent operation of TPVs would both allow the generation of electricity when the use of noisy conventional generators is not allowed, and not disturb others when the use of generators is permitted. However, the emitter temperatures required for practical efficiencies make TPVs on this scale extremely unlikely.[17]
References
- ^ Poortmans, Jef. "IMEC website: Photovoltaic Stacks". Archived from the original on 2007-10-13. http://web.archive.org/web/20071013061056/http://www.imec.be/wwwinter/energy/space_main.shtml. Retrieved 2008-02-17.
- ^ Seal, M.R.. "WWU VRI website: Viking 29 – A Thermophotovoltaic Hybrid Vehicle Designed and Built at Western Washington University". http://vri.etec.wwu.edu/tpv_paper.html. Retrieved 2010-11-12.
- ^ a b Nelson, R.E. (2003). "A brief history of thermophotovoltaic development". Semiconductor Science and Technology 18 (5): S141–S143. Bibcode 2003SeScT..18S.141N. doi:10.1088/0268-1242/18/5/301.
- ^ Horne E. (2002). Hybrid thermophotovoltaic power systems. Final report by EDTEK Inc. for the California energy commission.
- ^ Bitnar, B. (2003). "Silicon, germanium and silicon/germanium photocells for thermophotovoltaics applications". Semiconductor Science and Technology 18 (5): S221. doi:10.1088/0268-1242/18/5/312. http://klaihem.tripod.com/Aoucher_aSiGe/bitnar.pdf.
- ^ Malyshev, V. I. (1979). Introduction to Experimental Spectroscopy (in Russian) Nauka, Moscow.
- ^ Lin, S. Y., Moreno, J. and Fleming, J. G. (2003). "Three-dimensional photonic-crystal emitter for thermal photovoltaic power generation". Applied Physics Letters 83 (2): 380. doi:10.1063/1.1592614.
- ^ a b Fraas, L.M., Avery, J.E., Sundaram, V.S., Dinh, V.T., Davenport, T.M. and Yerkes, J.W. (1990). "Over 35% efficient GaAs/GaSb stacked concentrator cell assemblies for terrestrial applications". IEEE Conference on Photovoltaic Specialists. pp. 190. doi:10.1109/PVSC.1990.111616.
- ^ Algora, C. and Martin, D. (2003). "Modelling And Manufacturing GaSb TPV Converters". Modelling and Manufacturing GaSb TPV. 653. pp. 452. Bibcode 2003AIPC..653..452A. doi:10.1063/1.1539400.
- ^ a b Charache, G. W.; Egley, J. L.; Depoy, D. M.; Danielson, L. R.; Freeman, M. J.; Dziendziel, R. J.; Moynihan, J. F.; Baldasaro, P. F. et al. (1998). "Infrared Materials for Thermophotovoltaic Applications". Journal of Electronic Materials 27 (9): 1038. doi:10.1007/s11664-998-0160-x.
- ^ a b c Wang, C.A. (2004). "Antimony-Based III–V Thermophotovoltaic Materials and Devices". Antimony-based III-V thermophotovoltaic materials and devices. 738. pp. 255. Bibcode 2004AIPC..738..255W. doi:10.1063/1.1841902.
- ^ Karlina, L.B., Kulagina, M.M., Timoshina, N.Kh., Vlasov, A.S. and Andreev, V.M. (2007). "In0.53Ga0.47As/InP conventional and inverted thermophotovoltaic cells with back surface reflector". In0.53Ga0.47As/InP conventional and inverted thermophotovoltaic cells with back surface reflector. 890. pp. 182. Bibcode 2007AIPC..890..182K. doi:10.1063/1.2711735.
- ^ Guazzoni, G. and Matthews, S. (2004). "A Retrospective of Four Decades of Military Interest in Thermophotovoltaics". AIP Conference Proceedings. 738. pp. 3. Bibcode 2004AIPC..738....3G. doi:10.1063/1.1841874.
- ^ a b Teofilo, V.L., Choong, P., Chang, J., Tseng, Y.L., and Ermer, S. (2008). "Thermophotovoltaic Energy Conversion for Space". Journal of Physical Chemistry C 112 (21): 7841. doi:10.1021/jp711315c.
- ^ a b Wilt, D., Chubb, D., Wolford, D., Magari, P. and Crowley, C. (2007). "Thermophotovoltaics for Space Power Applications". AIP Conference Proceedings. 890. pp. 335. Bibcode 2007AIPC..890..335W. doi:10.1063/1.2711751.
- ^ Palfinger, G., Bitnar, B., Durisch, W., Mayor, J.C., Grützmacher, D. and Gobrecht, J. (2003). "Cost estimate of electricity produced by TPV". Semiconductor Science and Technology 18 (5): S254. doi:10.1088/0268-1242/18/5/317.
- ^ Coutts, T. J. (1997). "Thermophotovoltaic principles, potential, and problems". AIP Conference Proceedings. pp. 217. Bibcode 1997AIPC..404..217C. doi:10.1063/1.53449.
External links
- Thermophotovoltaics at Western Washington University.
- Thermophotovoltaics at Massachusetts Institute of Technology.
- Thermophotovoltaics at the Ioffe Institute.
- 6th International Conference on Thermophotovoltaic Generation of Electricity
- NASA Radioisotope Power Conversion Technology NRA Overview
- New thermophotovoltaic materials could replace alternators in cars and save fuel