Thermoelectric materials
| Thermoelectric effect |
|---|
|
Applications
Thermoelectric materials · Thermocouple · Thermopile · Thermoelectric cooling · Thermoelectric generator · Radioisotope thermoelectric generator · Automotive thermoelectric generator
|
Thermoelectric materials show the thermoelectric effect in a strong and/or convenient form. The thermoelectric effect refers to phenomena by which either a temperature difference creates an electric potential or an electric potential creates a temperature difference. These phenomena are known more specifically as the Seebeck effect (temperature->current), Peltier effect (current->temperature), and Thomson effect (conductor heating/cooling). While all materials have a nonzero thermoelectric effect, in most materials it is too small to be useful. However, low-cost materials that have a sufficiently strong thermoelectric effect (and other required properties) could be used in applications including power generation and refrigeration.
A commonly used thermoelectric material in such applications is bismuth telluride (Bi2Te3).
Contents |
Applications
Power generation
Approximately 90% of the world’s electricity is generated by heat energy, typically operating at 30-40% efficiency, losing roughly 15 terawatts of power in the form of heat to the environment. Thermoelectric devices could convert some of this waste heat into useful electricity.[1] Thermoelectric efficiency depends on the figure of merit, ZT. There is no theoretical upper limit to ZT, and as ZT approaches infinity, the thermoelectric efficiency approaches the Carnot limit. However, no known thermoelectrics have a ZT>3.[2] As of 2010, thermoelectric generators serve application niches where efficiency and cost are less important than reliability, light weight, and small size.[3]
Internal combustion engines capture 20-25% of the energy released during fuel combustion.[4] Increasing the conversion rate can increase mileage and provide more electricity for on-board controls and creature comforts (stability controls, telematics, navigation systems, electronic braking, etc.)[5] It may be possible to shift energy draw from the engine (in certain cases) to the electrical load in the car, e.g. electrical power steering or electrical coolant pump operation.[4]
Cogeneration power plants use the heat produced during electricity generation for alternative purposes. Thermoelectrics may find applications in such systems or in solar thermal energy generation.[6]
Refrigeration
Thermoelectric materials can be used as refrigerators, called "thermoelectric coolers", or "Peltier coolers" after the Peltier effect that controls their operation. As a refrigeration technology, Peltier cooling is far less common than vapor-compression refrigeration. The main advantages of a Peltier cooler (compared to a vapor-compression refrigerator) are its lack of moving parts or circulating fluid, and its small size and flexible shape (form factor). Another advantage is that Peltier coolers do not require refrigerant fluids, such as chlorofluorocarbons (CFCs) and related chemicals, which can have harmful environmental effects.[7]
The main disadvantage of Peltier coolers is that they cannot simultaneously have low cost and high power efficiency. Advances in thermoelectric materials may allow the creation of Peltier coolers that are both cheap and efficient. It is estimated that materials with ZT>3 (about 20-30% Carnot efficiency) are required to replace traditional coolers in most applications.[8] Today, Peltier coolers are only used in niche applications.
Materials selection criteria
Figure of merit
The primary criterion for thermoelectric device viability is the figure of merit given by:
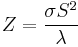 ,
,
which depends on the Seebeck coefficient, 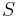 , thermal conductivity,
, thermal conductivity, 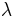 , and electrical conductivity,
, and electrical conductivity, 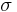 . The product (ZT) of Z and the use temperature, T, serves as a dimensionless parameter to evaluate the performance of a thermoelectric material.
. The product (ZT) of Z and the use temperature, T, serves as a dimensionless parameter to evaluate the performance of a thermoelectric material.
Phonon-Glass, electron-crystal behavior
Notably, in the above equation, thermal conductivity and electrical conductivity intertwine. G. A. Slack[9] proposed that in order to optimize the figure of merit, phonons, which are responsible for thermal conductivity must experience the material as they would in a glass (experiencing a high degree of phonon scattering—lowering thermal conductivity) while electrons must experience it as a crystal (experiencing very little scattering—maintaining electrical conductivity). The figure of merit can be improved through the independent adjustment of these properties.
Semiconductors
Semiconductors are ideal thermoelectric devices because of their band structure and electronic properties at high temperatures. Device efficiency is proportional to ZT, so ideal materials have a large Z value at high temperatures. Since temperature is easily adjustable, electrical conductivity is crucial. Specifically, maximizing electrical conductivity at high temperatures and minimizing thermal conductivity optimizes ZT.
Thermal conductivity
κ = κ electron + κ phonon
According to the Wiedemann–Franz law, the higher the electrical conductivity, the higher κ electron becomes.[10] Therefore, it is necessary to minimize κ phonon. In semiconductors, κ electron < κ phonon, so it is easier to decouple κ and σ in a semiconductor through engineering κ phonon.
Electrical conductivity
Metals are typically good electrical conductors, but the higher the temperature, the lower the conductivity, given by the equation for electrical conductivity:
σmetal = ne2τ/m
- n is carrier density
- e is electron charge
- τ is electron lifetime
- m is mass
As temperature increases, τ decreases, thereby decreasing σmetal. By contrast, electrical conductivity in semiconductors correlates positively with temperature.
σ semiconductor = neμ
- n is carrier density
- e is electron charge
- μ is carrier mobility
Carrier mobility decreases with increasing temperature, but carrier density increases faster with increasing temperature, resulting in increasing σ semiconductor.[11]
State density
The band structure of semiconductors offers better thermoelectric effects than the band structure of metals.
The Fermi energy is below the conduction band causing the state density to be asymmetric around the Fermi energy. Therefore, the average electron energy is higher than the Fermi energy, making the system conducive for charge motion into a lower energy state. By contrast, the Fermi energy lies in the conduction band in metals. This makes the state density symmetric about the Fermi energy so that the average conduction electron energy is close to the Fermi energy, reducing the forces pushing for charge transport. Therefore, semiconductors are ideal thermoelectric materials.[10]
Materials of interest
Strategies to improve thermoelectrics include both advanced bulk materials and the use of low-dimensional systems. Such approaches to reduce lattice thermal conductivity fall under three general material types: (1) Alloys: create point defects, vacancies, or rattling structures (heavy-ion species with large vibrational amplitudes contained within partially filled structural sites) to scatter phonons within the unit cell crystal.[12] (2) Complex crystals: separate the phonon-glass from the electron crystal using approaches similar to those for superconductors. The region responsible for electron transport would be an electron-crystal of a high-mobility semiconductor, while the phonon-glass would be ideal to house disordered structures and dopants without disrupting the electron-crystal (analogous to the charge reservoir in high-Tc superconductors.[13]) (3) Multiphase nanocomposites: scatter phonons at the interfaces of nanostructured materials,[14] be they mixed composites or thin film superlattices.
Materials under consideration for thermoelectric device applications include:
Bismuth chalcogenides
Materials such as Bi2Te3 and Bi2Se3 comprise some of the best performing room temperature thermoelectrics with a temperature-independent thermoelectric effect, ZT, between 0.8 and 1.0.[15] Nanostructuring these materials to produce a layered superlattice structure of alternating Bi2Te3 and Bi2Se3 layers produces a device within which there is good electrical conductivity but perpendicular to which thermal conductivity is poor. The result is an enhanced ZT (approximately 2.4 at room temperature for p-type).[16] Note that this high value has not entirely been independently confirmed.
Bismuth telluride and its solid solutions are good thermoelectric materials at room temperature and therefore suitable for refrigeration applications around 300 K. The Czochralski method has been used to grow single crystalline bismuth telluride compounds. These compounds are usually obtained with directional solidification from melt or powder metallurgy processes. Materials produced with these methods have lower efficiency than single crystalline ones due to the random orientation of crystal grains, but their mechanical properties are superior and the sensitivity to structural defects and impurities is lower due to high optimal carrier concentration.
The required carrier concentration is obtained by choosing a nonstoichiometric composition, which is achieved by introducing excess bismuth or tellurium atoms to primary melt or by dopant impurities. Some possible dopants are halogens and group IV and V atoms. Due to the small bandgap (0.16 eV) Bi2Te3 is partially degenerate and the corresponding Fermi-level should be close to the conduction band minimum at room temperature. The size of the band-gap means that Bi2Te3 has high intrinsic carrier concentration. Therefore, minority carrier conduction cannot be neglected for small stoichiometric deviations. Use of telluride compounds is limited by the toxicity and rarity of tellurium. [17](ch27)
Lead telluride
Jeffrey Snyder and his colleagues have shown in 2008 that with thallium doped lead telluride alloy (PbTe) it is possible to achieve zT of 1.5 at 773 K (Heremans et al., Science, 321(5888): 554-557). In an article published in January 2011, they showed that replacing thallium with Sodium zT~1.4 at 750 K is possible (Y. Pei et al., Energy Environ. Sci., 2011). In May 2011 they reported in Nature in collaboration with Chinese research group that PbTe1-xSex alloy doped with sodium gives zT~1.8±0.1 at 850 K (Y. Pei et al., Nature, 473 (5 May, 2011)). Snyder’s group has determined that both thallium and sodium alter the electronic structure of the crystal increasing electric conductivity. The Snyder group also claims that selenium increases further electric conductivity and also reduces thermal conductivity. These works show that other bulk alloys have also potential for improvement, which could open many new applications for thermoelectrics.
Inorganic clathrates
Inorganic clathrates have a general formula AxByC46-y (type I) and AxByC136-y (type II), in these formulas B and C are group III and IV atoms, respectively, which form the framework where “guest” atoms A (alkali or alkaline earth metal) are encapsulated in two different polyhedra facing each other. The differences between types I and II comes from number and size of voids present in their unit cells. Transport properties depend a lot on the properties of the framework, but tuning is possible through the “guest” atoms. [17](ch32-33) [18]
The most direct approach to the synthesis and optimization of thermoelectric properties of semiconducting type I clathrates is substitutional doping, where some framework atoms are replaced with dopant atoms. In addition, powder metallurgical and crystal growth techniques have been used in the synthesis of clathrates. The structural and chemical properties of clathrates enable the optimization of their transport properties with stoichiometry. Type II materials should be investigated in future because their structure allows a partial filling of the polyhedron enabling a better tuning of the electrical properties and therefore a better control of the doping level. Partially filled variant can also be synthesized as semiconducting or even insulating.
Blake et al have predicted zT~0.5 at room temperature and zT~1.7 at 800 K for optimized compositions. Kuznetsov et al measured electrical resistance and Seebeck coefficient for three different type I clathrates above room temperature and by estimating high temperature thermal conductivity from the published low temperature data they obtained zT~0.7 at 700 K for Ba8Ga16Ge30 and zT~0.87 at 870 K for Ba8Ga16Si30. [17](ch32-33)
Magnesium group IV compounds
Mg2BIV (BIV=Si, Ge, Sn) compounds and their solid solutions are good thermoelectric materials and their figure of merit values are comparable with those of established materials. Due to a lack of systematic studies about their thermoelectric properties, however, the suitability of these materials, and in particular their quasi-ternary solutions, for thermoelectric energy conversion remains in question. The appropriate production methods are based on direct co-melting, but mechanical alloying has also been used. During synthesis, magnesium losses due to evaporation and segregation of components (especially for Mg2Sn) need to be taken into account. Directed crystallization methods can produce single crystalline material. Solid solutions and doped compounds have to be annealed in order to produce homogeneous samples. At 800 K Mg2Si1-xSnx has been reported to have a figure of merit about 0.9. [17](ch29)
Silicides
Higher silicides seem promising materials for thermoelectric energy conversion, because their figure of merit is at the level with materials currently in use and they are mechanically and chemically strong and therefore can often be used in harsh environments without any protection. More detailed studies are needed to assess their potential in thermoelectrics and possibly to find a way to increase their figure of merit. Some of possible fabrication methods are Czochralski and floating zone for single crystals and hot pressing and sintering for polycrystalline. [19] (ch31)
Skutterudite thermoelectrics
Recently, skutterudite materials have sparked the interest of researchers in search of new thermoelectrics[20] These structures are of the form (Co,Ni,Fe)(P,Sb,As)3 and are cubic with space group Im3. Unfilled, these materials contain voids into which low-coordination ions (usually rare earth elements) can be inserted in order to alter thermal conductivity by producing sources for lattice phonon scattering and decrease thermal conductivity due to the lattice without reducing electrical conductivity.[21] Such qualities make these materials exhibit PGEC behavior.
The composition of skutterudites corresponds to the chemical formula LM4X12, where L is a rare earth metal, M a transition metal and X a metalloid, a group V element or pnictogen whose properties lie between those of a metal and nonmetal such as phosphorus, antimony, or arsenic. These materials could be potential in multistage thermoelectric devices as it has been shown that they have zT>1.0, but their properties are not well known and optimization of their structures is under way. [17](ch34)
Oxide thermoelectrics
Because of their layered superlattice structure, homologous oxide compounds (such as those of the form (SrTiO3)n(SrO)m—the Ruddleson-Popper phase) have the potential to be used in high-temperature thermoelectric devices.[22] These materials exhibit low thermal conductivity perpendicular to the layers while maintaining electrical conductivity within the layers. The figure of merit in oxides is still relatively low (~0.34 at 1,000K),[23], but their enhanced thermal stability, as compared to conventional high-ZT bismuth compounds, makes them superior for use in high-temperature applications.[24]
Interest in oxides as thermoelectric materials was reawakened in 1997 when NaxCoO2 was found to exhibit good thermoelectric behavior. In addition to their thermal stability, other advantages of oxides are their nontoxicity and high oxidation resistance. Research on thermoelectric oxide materials is ongoing, but it seems that in order to simultaneously control both the electric and phonon systems, nanostructured materials are required. Some layered oxide materials are thought to have zT~2.7 at 900 K. If the layers in a given material have the same stoichiometry, they will be stacked so that the same atoms will not be positioned on top of each other, impeding phonon conductivity perpendicular to the layers. [17](ch35)
Half Heusler alloys
Half Heusler alloys have potential for high temperature power generation applications especially as n-type material. These alloys have three components that originate from different element groups or might even be a combination of elements in the group. Two of the groups are composed of transition metals and the third group consists of metals and metalloids. Currently only n-type material is usable in thermoelectrics but some sources claim that they have achieved zT~1.5 at 700 K, but according to other source only zT~0.5 at 700 Khas been achieved. They state that primary reason for this difference is the disagreement between thermal conductivities measured by different groups. These alloys are relatively cheap and also have a high power factor. [25]
Electrically conducting organic materials
Some electrically conducting organic materials may have a higher figure of merit than existing inorganic materials. Seebeck coefficient can be even millivolts per Kelvin but electrical conductivity is usually very low resulting small figure of merit. Quasi one-dimensional organic crystals are formed from linear chains or stacks of molecules that are packed into a 3D crystal. It has theoretically been shown that under certain conditions some Q1D organic crystals may have zT~20 (Figure 13) at room temperature for both p- and n-type materials. In the Thermoelectrics Handbook chapter 36.4 this has been accredited to an unspecified interference between two main electron-phonon interactions leading to the formation of narrow strip of states in the conduction band with a significantly reduced scattering rate as the mechanism compensate each other causing high zT.[17](ch36)
Others
Silicon-germanium alloys are currently the best thermoelectric materials around 1000 ℃ and are therefore used in radioisotope thermoelectric generators (RTG) and some other high temperature applications, such as waste heat recovery. Usability of silicon-germanium alloys is limited by their high price and in addition, zT is also only in the mid-range (~0.7).
With functionally graded materials, it is possible to improve the conversion efficiency of existing thermoelectric materials. These materials have a non-uniform carrier concentration distribution and in some cases also solid solution composition. In power generation applications the temperature difference can be several hundred degrees and therefore devices made from homogeneous materials have some part that operates at the temperature where zT is substantially lower than its maximum value. This problem can be solved by using materials whose transport properties vary along their length thus enabling substantial improvements to the operating efficiency over large temperature differences. This is possible with functionally graded materials as they have a variable carrier concentration along the length of the material, which is optimized for operations over specific temperature range.[17](ch38)
Nanomaterials
In addition to the nanostructured Bi2/Bi2Se3 superlattice thin films that have shown a great deal of promise, other nanomaterials show potential in improving thermoelectric materials. One example involving PbTe/PbSeTe quantum dot superlattices provides an enhanced ZT (approximately 1.5 at room temperature) that was higher than the bulk ZT value for either PbTe or PbSeTe (approximately 0.5).[8] Individual silicon nanowires can act as efficient thermoelectric materials, with ZT values approaching 1.0 for their structures, even though bulk silicon is a poor thermoelectric material (approximately 0.01 at room temperature) because of its high thermal conductivity.[1][26]
Not all nanocrystalline materials are stable, because the crystal size can grow at high temperatures ruining materials desired characteristics. In nanocrystalline material, there are many interfaces between crystals, which scatter phonons so the thermal conductivity is reduced. Phonons are confined to the grain, if their mean free path is larger than the material grain size. Measured lattice thermal conductivity in nanowires is known to depend on roughness, the method of synthesis and properties of the source material. [27]
Nanocrystalline transition metal silicides are a promising material group for thermoelectric applications, because they fulfill several criteria that are demanded from the commercial applications point of view. In some nanocrystalline transition metal silicides the power factor is higher than in the corresponding polycrystalline material but the lack of reliable data on thermal conductivity prevents the evaluation of their thermoelectric efficiency.[17] (ch40)
One advantage of nanostructured skutterudites over normal skutterudites is their reduced thermal conductivity but further performance improvements can be achieved by using composites and by controlling the grain size, the compaction conditions of polycrystalline samples and the carrier concentration. Thermal conductivity reduction is caused by grain boundary scattering. ZT values of ~ 0.65 and >0.4 have been achieved with CoSb3 based samples, the former value is for 2.0 at.% Ni and 0.75 at.% Te doped material at 680 K and latter for Au-composite at T>700 K. [17](ch41)
Due to the unique nature of graphene, engineering of thermoelectric device with extremely high Seebeck coefficient based on this material is possible. One theoretical study suggests that the Seebeck coefficient might achieve a value of 30 mV/K at room temperature and zT for their proposed device would be approximately 20. [28]
Superlattices and quantum wells can be good thermoelectric materials, but their production is too difficult and expensive for general use because of their fabrication is based on various thin film growth methods. Superlattice structures allow the independent manipulation of transport parameters by adjusting the structural parameters enabling the search for better understanding of thermoelectric phenomena in nanoscale. Many strategies exist to decrease the superlattice thermal conductivity that are based on engineering of phonon transport. The thermal conductivity along the film plane and wire axis can be reduced by creating diffuse interface scattering and by reducing the interface separation distance, both which are caused by interface roughness. The interface roughness can be natural due to the mixing of atoms at the interfaces or artificial. Many different structure types, such as quantum dot interfaces and thin films on step-covered substrates, can act as source for artificial roughness. [29]
However while engineering interface structures for reduced phonon thermal conductivity effects to electron transport has to be taken into account because the reduced electrical conductivity could negate the advantage received from phonon transport engineering. Because electrons and phonons have different wavelengths, it may be possible to engineer the structure in such a way that phonons are scattered more diffusely at the interface than electrons. This would reduce the decrease of the electrical conductivity.
Second approach is to increase phonon reflectivity and therefore decrease the thermal conductivity perpendicular to interfaces. This can be achieved by increasing the mismatch between the materials. Some of these properties are density, group velocity, specific heat, and the phonon spectrum between adjacent layers. Interface roughness causes diffuse phonon scattering, which either increases or decreases the phonon reflectivity at the interfaces. Mismatch between bulk dispersion relations confines phonons and the confinement becomes more favorable as the difference in dispersion increases. The amount of confinement is currently unknown as only some models and experimental data exist. As with a previous method, the effects on the electrical conductivity have to be considered. [29]
In order to further reduce the thermal conductivity, the localization of long wavelength phonons can be attempted with aperiodic superlattices or composite superlattices with different periodicities. In addition, defects, especially dislocations, can be used to reduce thermal conductivity in low dimensional systems. [29]
Thermoelectric performance improvements in superlattices originate from various sources, usually at least the lattice thermal conductivity in the cross plane direction is very low but depending on the type of superlattice, the thermoelectric coefficient may also increase because the bandstructure changes. Low lattice thermal conductivity in superlattices is usually due to strong interface scattering of phonons. Electronic bandstructure in superlattices comprises the so called minibands, which appear due to quantum confinement effects. In superlattices, electronic bandstructure depends on the superlattice period so that with very short period (~1 nm) the bandstructure approaches the alloy limit and with long period (≥ ~60 nm) minibands become so close to each other that they can be approximated with a continuum. [17](ch16, 39)
Especially in multi quantum well structures the parasitic heat conduction could cause significant performance reduction. Fortunately, the impact of this phenomenon can be reduced by choosing the distance between the quantum wells correctly.
The Seebeck coefficient can change its sign in superlattice nanowires due to the existence of minigaps as Fermi energy varies. This indicates that superlattices can be tailored to exhibit n or p-type behavior by using the same dopants as those that are used for corresponding bulk materials by carefully controlling Fermi energy or the dopant concentration. With nanowire arrays, it is possible to exploit semimetal-semiconductor transition due to the quantum confinement and use materials that normally would not be good thermoelectric materials in bulk form. Such elements are for example bismuth. The Seebeck effect could also be used to determine the carrier concentration and Fermi energy in nanowires.[17](ch39)
In quantum dot thermoelectrics, unconventional or nonband transport behavior (e.g. tunneling or hopping) is necessary to utilize their special electronic bandstructure in the transport direction. It is possible to achieve zT~3 at elevated temperatures with quantum dot superlattices, but they are almost always unsuitable for mass production. Bi2Te3/Sb2Te3 superlattice as a microcooler has been reported to have zT~2.4 at 300 K. [17](ch49)
Nanocomposites are promising material class for bulk thermoelectric devices, but several challenges have to be overcome to make them suitable for practical applications. It is not well understood why the improved thermoelectric properties appear only in certain materials with specific fabrication processes.[30]
K. Biswas et al. report in Nature Chemistry 3, 160–166 (2011) that SrTe nanocrystals embedded in a bulk PbTe matrix so that rocksalt lattices of both materials are completely aligned (endotaxy) with optimal molar concentration for SrTe only 2 % can cause strong phonon scattering but would not affect charge transport. They report maximum zT~1.7 at 815 K for p-type material.
Production methods
Production methods for these materials can be divided into powder and crystal growth based techniques. Powder based techniques offer excellent ability to control and maintain desired carrier distribution. In crystal growth techniques dopants are often mixed with melt, but diffusion from gaseous phase can also be used. In the zone melting techniques disks of different materials are stacked on top of others and then materials are mixed with each other when a travelling heater causes melting. In powder techniques, either different powders are mixed with a varying ratio before melting or they are in different layers as a stack before pressing and melting.
See also
- Thermoelectric effect
- Thermopower
- Batteryless radio
- Joule's law
- Heat transfer
- Thermoelectric cooling/ Peltier device
- Pyroelectric effect
- Thermogenerator
- Thermionic emission
- Bismuth telluride
References
- ^ a b Hochbaum, A. I. et al. Enhanced thermoelectric performance of rough silicon nanowires. Nature 451, 163–167 (2008).
- ^ Tritt, T. M.; Subramanian, M. A., Thermoelectric Materials, Phenomena, and Applications: A Bird's Eye View. MRS Bulletin 2006, 31, (3), 188-194.
- ^ M. Labudovic and J. Li, “Modeling of TE cooling of pump lasers,” IEEE Trans. Compon. Packag. Tech., vol. 27, p. 724, 2004.
- ^ a b Yang, International Conference on Thermoelectrics: 2005, pp. 155.
- ^ Fairbanks, J., Thermoelectric Developments for Vehicular Applications, U.S. Department of Energy: Energy Efficiency and Renewable Energy. Presented on: August 24, 2006.
- ^ Tritt et al., "Thermoelectrics: Direct Solar Thermal Energy Conversion," MRS Bulletin: April 2008, Vol. 33, pp. 366-8
- ^ NOAA: Earth System Research Laboratory, Hydrochlorofluorocarbon measurements in the Chlorofuorocarbon Alternatives Measurement Project, http://www.esrl.noaa.gov/gmd/hats/flask/hcfc.html.
- ^ a b Harman, T. C.; Taylor, P. J.; Walsh, M. P.; LaForge, B. E., Quantum dot superlattice thermoelectric materials and devices. Science 2002, 297, (5590), 2229-2232.
- ^ Slack GA., CRC Handbook of Thermoelectrics, ed. DM Rowe, Boca Raton, FL: CRC Press (1995)
- ^ a b Timothy D. Sands (2005), "Designing Nanocomposite Thermoelectric Materials," http://nanohub.org/resources/383.
- ^ , N.W. Ashcroft and N.D. Mermin, Solid State Physics, (Holt, Rinehart, and Winston, New York, 1976).
- ^ Bhandari, C. M. in CRC Handbook of Thermoelectrics (ed. Rowe, D. M.) 55–65 (CRC, Boca Raton, 1995).
- ^ Cava, R. J. Structural chemistry and the local charge picture of copper-oxide superconductors. Science 247, 656–662 (1990).
- ^ Dresselhaus, M. S. et al. New directions for low-dimensional thermoelectric materials. Adv. Mater. 19, 1043–1053 (2007).
- ^ D.Y. Chung et al., Complex Bismuth Chalcogenides as Thermoelectrics, 16th International Conference on Thermoelectrics (1997), pp. 459-462
- ^ Venkatasubramanian et al., Nature, 413, 597 (2001)
- ^ a b c d e f g h i j k l m Rowe, David Michael. Thermoelectrics handbook : macro to nano / edited by D.M. Rowe. Boca Raton: CRC/Taylor & Francis, 2006. ISBN 0-8493-2264-2
- ^ Gatti, C., Bertini, L., Blake, N. P. and Iversen, B. B. (2003), Guest–Framework Interaction in Type I Inorganic Clathrates with Promising Thermoelectric Properties: On the Ionic versus Neutral Nature of the Alkaline-Earth Metal Guest A in A8Ga16Ge30 (A=Sr, Ba). Chemistry -A European Journal, 9: 4556–4568. doi: 10.1002/chem.200304837
- ^ Rowe, David Michael. Thermoelectrics handbook : macro to nano / edited by D.M. Rowe. Boca Raton: CRC/Taylor & Francis, 2006. ISBN 0-8493-2264-2
- ^ Caillat, T., Borshchevsky, A., and Fleurial, J.-P., In Proceedings of 7th International Conference TEs, K. Rao, ed., pp. 98 – 101. University of Texas, Arlington, 1993.
- ^ Nolas et al., J. Appl. Phys ., 79 (1996), 4002-8
- ^ K. Koumoto,I. Terasaki, T. Kajitani, M. Ohtaki, R. Funahashi “Oxide Thermoelectrics”; Section 35: pp.1-14 in Thermoelectrics Handbook: Macro to Nano, Edited by D.M. Rowe, CRC Press: New York (2006).
- ^ W. Wunderlich, S. Ohta, and K. Koumoto, 24th International Conference on Thermoelectrics, 2005, pp. 252-255
- ^ M. Senthilkumar et al. "High-temperature resistivity and thermoelectric properties of coupled substituted Ca3Co2O6" Sci. Technol. Adv. Mater. 10 (2009) 015007 free download
- ^ Slade R. Culp, S. Joseph Poon, Nicoleta Hickman, Terry M. Tritt, and J. Blumm, Effect of substitutions on the thermoelectric figure of merit of half-Heusler phases at 800 °C, Appl. Phys. Lett. 88, 042106 (2006), DOI:10.1063/1.2168019
- ^ A.I. Boukai et al. "Silicon nanowires as efficient thermoelectric materials" Nature 451 (2007) 168-171
- ^ Paothep Pichanusakorn, Prabhakar Bandaru. Nanostructured thermoelectrics, Materials Science and Engineering: R: Reports, Volume 67, Issues 2-4, 29 January 2010, Pages 19-63, ISSN 0927-796X, DOI: 10.1016/j.mser.2009.10.001.
- ^ 16 D. Dragoman and M. Dragoman, Giant thermoelectric effect in graphene, Appl. Phys. Lett. 91, 203116 (2007), DOI:10.1063/1.2814080
- ^ a b c Chen, G.; Dresselhaus, M. S.; Dresselhaus, G.; Fleurial, J.-P.; Caillat, T. Recent developments in thermoelectric materials, International Materials Reviews, Volume 48, Number 1, February 2003, pp. 45-66(22)
- ^ A. J. Minnich, M. S. Dresselhaus, Z. F. Ren and G. Chen. Bulk nanostructured thermoelectric materials: current research and future prospects, Energy & Environmental Science, 2009, 2, 466-479, DOI: 10.1039/b822664b