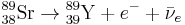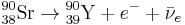Isotopes of strontium
The alkaline earth metal strontium (Sr) has four stable, naturally occurring isotopes: 84Sr (0.56%), 86Sr (9.86%), 87Sr (7.0%) and 88Sr (82.58%). It has a standard atomic mass of 87.62(1) u.
Only 87Sr is radiogenic; it is produced by decay from the radioactive alkali metal 87Rb, which has a half-life of 4.88 × 1010 years. Thus, there are two sources of 87Sr in any material: that formed during primordial nucleo-synthesis along with 84Sr, 86Sr and 88Sr, as well as that formed by radioactive decay of 87Rb. The ratio 87Sr/86Sr is the parameter typically reported in geologic investigations; ratios in minerals and rocks have values ranging from about 0.7 to greater than 4.0. Because strontium has an electron configuration similar to that of calcium, it readily substitutes for Ca in minerals.
Twenty-nine Thirty-one unstable isotopes are known to exist, the longest-lived of which are 90Sr with a half-life of 28.9 years and 85Sr with a half-life of 64.853 days. Of importance are strontium-89 (89Sr) with a half-life of 50.57 days, and strontium-90 (90Sr). They decay by emitting an electron and an anti-neutrino (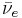 ) in beta decay (β− decay) to become yttrium:
) in beta decay (β− decay) to become yttrium:
89Sr is an artificial radioisotope which is used in treatment of bone cancer. In circumstances where cancer patients have widespread and painful bony metastases, the administration of 89Sr results in the delivery of beta particles directly to the area of bony problem, where calcium turnover is greatest.
90Sr is a by-product of nuclear fission which is found in nuclear fallout and presents a health problem since it substitutes for calcium in bone, preventing expulsion from the body. Because it is a long-lived high-energy beta emitter, it is used in SNAP (Systems for Nuclear Auxiliary Power) devices. These devices hold promise for use in spacecraft, remote weather stations, navigational buoys, etc., where a lightweight, long-lived, nuclear-electric power source is required. The 1986 Chernobyl nuclear accident contaminated a vast area with 90Sr.
The lightest isotope is 73Sr and the heaviest being 107Sr.
All other isotopes have half-lives shorter than 55 days, most under 100 minutes.
Table
| nuclide symbol |
Z(p) | N(n) | isotopic mass (u) |
half-life | decay mode(s)[1][n 1] |
daughter isotope(s)[n 2] |
nuclear spin |
representative isotopic composition (mole fraction) |
range of natural variation (mole fraction) |
|---|---|---|---|---|---|---|---|---|---|
| excitation energy | |||||||||
| 73Sr | 38 | 35 | 72.96597(64)# | >25 ms | β+ (>99.9%) | 73Rb | 1/2-# | ||
| β+, p (<.1%) | 72Kr | ||||||||
| 74Sr | 38 | 36 | 73.95631(54)# | 50# ms [>1.5 µs] | β+ | 74Rb | 0+ | ||
| 75Sr | 38 | 37 | 74.94995(24) | 88(3) ms | β+ (93.5%) | 75Rb | (3/2-) | ||
| β+, p (6.5%) | 74Kr | ||||||||
| 76Sr | 38 | 38 | 75.94177(4) | 7.89(7) s | β+ | 76Rb | 0+ | ||
| 77Sr | 38 | 39 | 76.937945(10) | 9.0(2) s | β+ (99.75%) | 77Rb | 5/2+ | ||
| β+, p (.25%) | 76Kr | ||||||||
| 78Sr | 38 | 40 | 77.932180(8) | 159(8) s | β+ | 78Rb | 0+ | ||
| 79Sr | 38 | 41 | 78.929708(9) | 2.25(10) min | β+ | 79Rb | 3/2(-) | ||
| 80Sr | 38 | 42 | 79.924521(7) | 106.3(15) min | β+ | 80Rb | 0+ | ||
| 81Sr | 38 | 43 | 80.923212(7) | 22.3(4) min | β+ | 81Rb | 1/2- | ||
| 82Sr | 38 | 44 | 81.918402(6) | 25.36(3) d | EC | 82Rb | 0+ | ||
| 83Sr | 38 | 45 | 82.917557(11) | 32.41(3) h | β+ | 83Rb | 7/2+ | ||
| 83mSr | 259.15(9) keV | 4.95(12) s | IT | 83Sr | 1/2- | ||||
| 84Sr | 38 | 46 | 83.913425(3) | Observationally Stable[n 3] | 0+ | 0.0056(1) | 0.0055-0.0058 | ||
| 85Sr | 38 | 47 | 84.912933(3) | 64.853(8) d | EC | 85Rb | 9/2+ | ||
| 85mSr | 238.66(6) keV | 67.63(4) min | IT (86.6%) | 85Sr | 1/2- | ||||
| β+ (13.4%) | 85Rb | ||||||||
| 86Sr | 38 | 48 | 85.9092602(12) | Stable | 0+ | 0.0986(1) | 0.0975-0.0999 | ||
| 86mSr | 2955.68(21) keV | 455(7) ns | 8+ | ||||||
| 87Sr[n 4] | 38 | 49 | 86.9088771(12) | Stable | 9/2+ | 0.0700(1) | 0.0694-0.0714 | ||
| 87mSr | 388.533(3) keV | 2.815(12) h | IT (99.7%) | 87Sr | 1/2- | ||||
| EC (.3%) | 87Rb | ||||||||
| 88Sr[n 5] | 38 | 50 | 87.9056121(12) | Stable | 0+ | 0.8258(1) | 0.8229-0.8275 | ||
| 89Sr[n 5] | 38 | 51 | 88.9074507(12) | 50.57(3) d | β- | 89Y | 5/2+ | ||
| 90Sr[n 5] | 38 | 52 | 89.907738(3) | 28.90(3) a | β- | 90Y | 0+ | ||
| 91Sr | 38 | 53 | 90.910203(5) | 9.63(5) h | β- | 91Y | 5/2+ | ||
| 92Sr | 38 | 54 | 91.911038(4) | 2.66(4) h | β- | 92Y | 0+ | ||
| 93Sr | 38 | 55 | 92.914026(8) | 7.423(24) min | β- | 93Y | 5/2+ | ||
| 94Sr | 38 | 56 | 93.915361(8) | 75.3(2) s | β- | 94Y | 0+ | ||
| 95Sr | 38 | 57 | 94.919359(8) | 23.90(14) s | β- | 95Y | 1/2+ | ||
| 96Sr | 38 | 58 | 95.921697(29) | 1.07(1) s | β- | 96Y | 0+ | ||
| 97Sr | 38 | 59 | 96.926153(21) | 429(5) ms | β- (99.95%) | 97Y | 1/2+ | ||
| β-, n (.05%) | 96Y | ||||||||
| 97m1Sr | 308.13(11) keV | 170(10) ns | (7/2)+ | ||||||
| 97m2Sr | 830.8(2) keV | 255(10) ns | (11/2-)# | ||||||
| 98Sr | 38 | 60 | 97.928453(28) | 0.653(2) s | β- (99.75%) | 98Y | 0+ | ||
| β-, n (.25%) | 97Y | ||||||||
| 99Sr | 38 | 61 | 98.93324(9) | 0.269(1) s | β- (99.9%) | 99Y | 3/2+ | ||
| β-, n (.1%) | 98Y | ||||||||
| 100Sr | 38 | 62 | 99.93535(14) | 202(3) ms | β- (99.02%) | 100Y | 0+ | ||
| β-, n (.98%) | 99Y | ||||||||
| 101Sr | 38 | 63 | 100.94052(13) | 118(3) ms | β- (97.63%) | 101Y | (5/2-) | ||
| β-, n (2.37%) | 100Y | ||||||||
| 102Sr | 38 | 64 | 101.94302(12) | 69(6) ms | β- (94.5%) | 102Y | 0+ | ||
| β-, n (5.5%) | 101Y | ||||||||
| 103Sr | 38 | 65 | 102.94895(54)# | 50# ms [>300 ns] | β- | 103Y | |||
| 104Sr | 38 | 66 | 103.95233(75)# | 30# ms [>300 ns] | β- | 104Y | 0+ | ||
| 105Sr | 38 | 67 | 104.95858(75)# | 20# ms [>300 ns] | |||||
- ^ Abbreviations:
EC: Electron capture
IT: Isomeric transition - ^ Bold for stable isotopes, bold italic for nearly-stable isotopes (half-life longer than the age of the universe)
- ^ Believed to decay by β+β+ to 84Kr
- ^ Used in rubidium-strontium dating
- ^ a b c Fission product
Notes
- Evaluated isotopic composition is for most but not all commercial samples.
- The precision of the isotope abundances and atomic mass is limited through variations. The given ranges should be applicable to any normal terrestrial material.
- Geologically exceptional samples are known in which the isotopic composition lies outside the reported range. The uncertainty in the atomic mass may exceed the stated value for such specimens.
- Values marked # are not purely derived from experimental data, but at least partly from systematic trends. Spins with weak assignment arguments are enclosed in parentheses.
- Uncertainties are given in concise form in parentheses after the corresponding last digits. Uncertainty values denote one standard deviation, except isotopic composition and standard atomic mass from IUPAC which use expanded uncertainties.
References
- Isotope masses from:
- G. Audi, A. H. Wapstra, C. Thibault, J. Blachot and O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties". Nuclear Physics A 729: 3–128. Bibcode 2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001. http://www.nndc.bnl.gov/amdc/nubase/Nubase2003.pdf.
- Isotopic compositions and standard atomic masses from:
- J. R. de Laeter, J. K. Böhlke, P. De Bièvre, H. Hidaka, H. S. Peiser, K. J. R. Rosman and P. D. P. Taylor (2003). "Atomic weights of the elements. Review 2000 (IUPAC Technical Report)". Pure and Applied Chemistry 75 (6): 683–800. doi:10.1351/pac200375060683. http://www.iupac.org/publications/pac/75/6/0683/pdf/.
- M. E. Wieser (2006). "Atomic weights of the elements 2005 (IUPAC Technical Report)". Pure and Applied Chemistry 78 (11): 2051–2066. doi:10.1351/pac200678112051. http://iupac.org/publications/pac/78/11/2051/pdf/. Lay summary.
- Half-life, spin, and isomer data selected from the following sources. See editing notes on this article's talk page.
- G. Audi, A. H. Wapstra, C. Thibault, J. Blachot and O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties". Nuclear Physics A 729: 3–128. Bibcode 2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001. http://www.nndc.bnl.gov/amdc/nubase/Nubase2003.pdf.
- National Nuclear Data Center. "NuDat 2.1 database". Brookhaven National Laboratory. http://www.nndc.bnl.gov/nudat2/. Retrieved September 2005.
- N. E. Holden (2004). "Table of the Isotopes". In D. R. Lide. CRC Handbook of Chemistry and Physics (85th ed.). CRC Press. Section 11. ISBN 978-0849304859.
| Isotopes of rubidium | Isotopes of strontium | Isotopes of yttrium |
| Index to isotope pages · Table of nuclides | ||