Serpentinite
Serpentinite is a rock composed of one or more serpentine group minerals. Minerals in this group are formed by serpentinization, a hydration and metamorphic transformation of ultramafic rock from the Earth's mantle. The alteration is particularly important at the sea floor at tectonic plate boundaries.
Contents |
Formation and petrology
Serpentinization is a geological low-temperature metamorphic process involving heat and water in which low-silica mafic and ultramafic rocks are oxidized (anaerobic oxidation of Fe2+ by the protons of water leading to the formation of H2) and hydrolyzed with water into serpentinite. Peridotite, including dunite, at and near the seafloor and in mountain belts is converted to serpentine, brucite, magnetite, and other minerals — some rare, such as awaruite (Ni3Fe), and even native iron. In the process large amounts of water are absorbed into the rock increasing the volume and destroying the structure.[1]
The density changes from 3.3 to 2.7 g/cm3 with a concurrent volume increase of about 40%. The reaction is exothermic and large amounts of heat energy are produced in the process.[1]
Rock temperatures can be raised by about 260 °C,[1] providing an energy source for formation of non-volcanic hydrothermal vents. The magnetite-forming chemical reactions produce hydrogen gas under anaerobic conditions prevailing deep in the mantle, far from the Earth atmosphere. Carbonates and sulfates are subsequently reduced by hydrogen and form methane and hydrogen sulfide. The hydrogen, methane, and hydrogen sulfide provide energy sources for deep sea chemotroph microorganisms.[1]
Serpentinite reactions
Serpentinite is formed from olivine via several reactions, some of which are complementary. Olivine is a solid solution between the magnesium-endmember forsterite and the iron-endmember fayalite. Serpentinite reactions 1a and 1b, below, exchange silica between forsterite and fayalite to form serpentine group minerals and magnetite. These are highly exothermic reactions.
Reaction 1a:
Fayalite + water → magnetite + aqueous silica + hydrogen
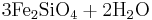 →
→ 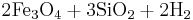
Reaction 1b:
Forsterite + aqueous silica → serpentine
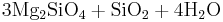 →
→ 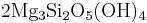
Reaction 1c:
Forsterite + water → serpentine + brucite
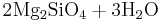 →
→ 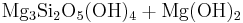
Reaction 1c describes the hydration of olivine with water only to yield serpentine and Mg(OH)2 (brucite). Serpentine is stable at high pH in the presence of brucite like calcium silicate hydrate, (C-S-H) phases formed along with portlandite (Ca(OH)2) in hardened Portland cement paste after the hydration of belite (Ca2SiO4), the artificial calcium equivalent of forsterite.
Analogy of reaction 1c with belite hydration in ordinary Portland cement:
Belite + water → C-S-H phase + portlandite
- 2 Ca2SiO4 + 4 H2O → 3 CaO · 2 SiO2 · 3 H2O + Ca(OH)2
After reaction, the poorly soluble reaction products (aqueous silica or dissolved magnesium ions) can be transported in solution out of the serpentinized zone by diffusion or advection.
A similar suite of reactions involves pyroxene-group minerals, though less readily and with complication of the additional end-products due to the wider compositions of pyroxene and pyroxene-olivine mixes. Talc and magnesian chlorite are possible products, together with the serpentine minerals antigorite, lizardite, and chrysotile. The final mineralogy depends both on rock and fluid compositions, temperature, and pressure. Antigorite forms in reactions at temperatures that can exceed 600°C during metamorphism, and it is the serpentine group mineral stable at the highest temperatures. Lizardite and chrysotile can form at low temperatures very near the Earth's surface. Fluids involved in serpentinite formation commonly are highly reactive and may transport calcium and other elements into surrounding rocks; fluid reaction with these rocks may create metasomatic reaction zones enriched in calcium and called rodingites.
In the presence of carbon dioxide, however, serpentinitization may form either magnesite (MgCO3) or generate methane (CH4). It is thought that some hydrocarbon gases may be produced by serpentinite reactions within the oceanic crust.
Reaction 2a:
- Olivine + water + carbonic acid → serpentine + magnetite + methane
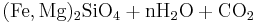 →
→ 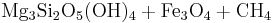
or, in balanced form:
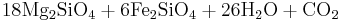 →
→ 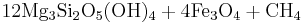
Reaction 2b:
- Olivine + water + carbonic acid → serpentine + magnetite + magnesite + silica
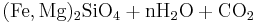 →
→ 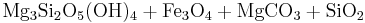
Reaction 2a is favored if the serpentinite is Mg-poor or if there isn't enough carbon dioxide to promote talc formation. Reaction 2b is favored in highly magnesian compositions and low partial pressure of carbon dioxide.
The degree to which a mass of ultramafic rock undergoes serpentinisation depends on the starting rock composition and on whether or not fluids transport calcium, magnesium and other elements away during the process. If an olivine composition contains sufficient fayalite, then olivine plus water can completely metamorphose to serpentine and magnetite in a closed system. In most ultramafic rocks formed in the Earth's mantle, however, the olivine is about 90% forsterite endmember, and for that olivine to react completely to serpentine, magnesium must be transported out of the reacting volume.
Serpentinitization of a mass of peridotite usually destroys all previous textural evidence because the serpentine minerals are weak and behave in a very ductile fashion. However, some masses of serpentinite are less severely deformed, as evidenced by the apparent preservation of textures inherited from the peridotite, and the serpentinites may have behaved in a rigid fashion.
Hydrogen production by anaerobic oxidation of fayalite ferrous ions
In the absence of atmospheric oxygen (O2), in deep geological conditions prevailing far away from Earth atmosphere, hydrogen (H2) is produced by the anaerobic oxidation of ferrous ions (Fe2+) present in the crystal lattice of the iron-endmember fayalite by the protons (H+) of water.[2][3]
Considering three formula units of fayalite (Fe2(SiO4)) for the purpose of stoechiometry and reaction mass balance, four ferrous ions will undergo oxidation by water protons while the two remaining will stay unoxidised. Neglecting the orthosilicate anions not involved in the redox process, it is then possible to schematically write the two half-redox reactions as follows:
- 4 (Fe2+ → Fe3+ + e–) (oxidation of ferrous ions)
- 2 (H2O + 2 e– → O2– + H2) (reduction of protons into hydrogen)
This leads to the global redox reaction involving ferrous ions oxidation by water:
- 4 Fe2+ + 2 H2O → 4 Fe3+ + 2 O2– + 2 H2
The two unoxidised ferrous (Fe2+) ions still available in the three formula units of fayalite finally combine with the four ferric (Fe3+) cations and oxide anions (O2–) to form two formula units of magnetite (Fe3O4).
Finally, considering the required rearrangement of the orthosilicate anions into free silica (SiO2) and free oxide anions (O2–), it is possible to write the complete reaction of anaerobic oxidation and hydrolysis of fayalite according to the following mass balance:
- 3 Fe2SiO4 + 2 H2O → 2 Fe3O4 + 3 SiO2 + 3 H2
- fayalite + water → magnetite + quartz + hydrogen
This reaction closely resembles the Schikorr reaction observed in the anaerobic oxidation of the ferrous hydroxide in contact with water:
- 3 Fe(OH)2 → Fe3O4 + 2 H2O + H2
- ferrous hydroxide → magnetite + water + hydrogen
Abiotic methane production on Mars by serpentinization
The presence of traces of methane in the atmosphere of Mars has been hypothesized to be a possible evidence for life on Mars if methane was produced by bacterial activity. Serpentinization has been proposed as an alternative non-biological source for the observed methane traces.[4][5]
Impact on agriculture
Soil cover over serpentinite bedrock tends to be thin or absent. Soil with serpentine is poor in calcium and other major plant nutrients, but rich in elements toxic to plants such as chromium and nickel.[6]
Uses for serpentinite
Decorative stone in architecture
Grades of serpentinite higher in calcite, along with the breccia form of serpentinite, have historically been used as decorative stones for their marble-like qualities. Popular sources in Europe before contact with the New World were the mountainous Piedmont region of Italy and Larissa, Greece.[7]
Swiss ovenstone
A variety of chlorite talc schist associated with Alpine serpentinite is found in Val d’Anniviers, Switzerland and was used as ovenstone in stove construction.[8]
Neutron shield in nuclear reactors
Serpentinite has a significant amount of bound water, hence it contains abundant hydrogen atoms able to slow down neutrons by elastic collision (neutron thermalization process). Because of this serpentinite can be used as dry filler inside steel jackets in some designs of nuclear reactors. For example in RBMK series it was used for top radiation shielding to protect operators from escaping neutrons.[9] Serpentine can also be added as aggregate to special concrete used in nuclear reactor shielding to increase the concrete density (2.6 g cm-3) and its neutron capture cross section.[10][11]
Cultural references
It is the state rock of California, USA and the California Legislature specified that serpentine was "the official State Rock and lithologic emblem."[12]
See also
- Serpentine group
- Serpentine soil, a soil derived from the serpentine mineral
- Schikorr reaction, involving also the formation of magnetite and hydrogen by a very similar mechanism
- Hydration of belite in cement (analogous to forsterite hydration)
- Cement chemist notation, also useful for silicate and oxide reactions in mineralogy
- Chrysotile dehydration
- Lost City (hydrothermal field)
- Common redox mineral buffer – FMQ: fayalite-magnetite-quartz
- Nephrite
- Carbon sequestration
- Talc carbonate
- Soapstone
References
- ^ a b c d Serpentinization: The heat engine at Lost City and sponge of the oceanic crust
- ^ "Methane and hydrogen formation from rocks – Energy sources for life". http://www.lostcity.washington.edu/science/chemistry/methane.html. Retrieved 2011-11-06.
- ^ Sleep, N.H.; A. Meibom, Th. Fridriksson, R.G. Coleman, D.K. Bird (2004). "H2-rich fluids from serpentinization: Geochemical and biotic implications". Proceedings of the National Academy of Sciences of the United States of America 101 (35): 12818–12823. doi:10.1073/pnas.0405289101. http://www.pnas.org/content/101/35/12818.abstract. Retrieved 2011-11-06.
- ^ "Life on Mars?". American Scientist. March–April 2006. http://www.americanscientist.org/issues/pub/life-on-mars. Retrieved 1 June 2009.
- ^ "Methane: Evidence of life on Mars?". redorbit.com. 15 January 2009. http://www.redorbit.com/news/space/1623762/methane_evidence_of_life_on_mars_update/index.html. Retrieved 1 June 2009.
- ^ "CVO Website - Serpentine and serpentinite", USGS/NPS Geology in the Parks Website, September 2001, accessed February 27, 2011.
- ^ Ashurst, John. Dimes, Francis G. Conservation of building and decorative stone. Elsevier Butterworth-Heinemann, 1990, p. 51.
- ^ Talcose-schist from Canton Valais. By Thomags Bonney, (Geol. Mag., 1897, N.S., [iv], 4, 110--116) abstract
- ^ Lithuanian Energy Institute (2011-05-28). "Design of structures, components, equipments and systems". Ignalina Source Book. http://www.lei.lt/insc/sourcebook/sob3/sob33.html. Retrieved 2011-05-28.
- ^ Aminian, A.; Nematollahi, M.R.; Haddad, K.; Mehdizadeh, S. (03-08 June 2007). "Determination of shielding parameters for different types of concretes by Monte Carlo methods". ICENES 2007: International Conference on Emerging Nuclear Energy Systems. Session 12B: Radiation effects. Istanbul, Turkey. pp. 7. http://www.icenes2007.org/icenes_proceedings/manuscripts.pdf/Session%2012B/DETERMINATION%20OF.pdf.
- ^ Abulfaraj, Waleed H.; Salah M. Kamal. "Evaluation of ilmenite serpentine concrete and ordinary concrete as nuclear reactor shielding". Radiation Physics and Chemistry 44 (1-2): 139–148. doi:16/0969-806X(94)90120-1. ISSN 0969-806X. http://www.sciencedirect.com/science/article/pii/0969806X94901201. Retrieved 2011-05-28.
- ^ California Government Code § 425.2; see http://www.leginfo.ca.gov/cgi-bin/displaycode?section=gov&group=00001-01000&file=420-429.8