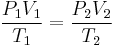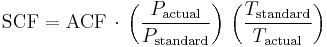Standard cubic feet per minute
Standard cubic feet per minute (SCFM) is the volumetric flow rate of a gas corrected to "standardized" conditions of temperature and pressure. It is equivalent to the molar flow rate by the ideal gas law. Conversion of SCFM to mass flow requires knowledge of the mixture averaged gas molecular weight. Consider that one mole of gas (STP) occupies about 22.414 liters. Thus, a one mole/second flow of hydrogen corresponds to 1g/s whereas a one mole/second flow of Krypton, MW=83.8, results in 83.8 g/s flow.
However, great care must be taken, as the "standard" conditions vary between definitions and should therefore always be checked. Worldwide, the "standard" condition for pressure is variously defined as an absolute pressure of 101,325 pascals, 1.0 bar (i.e., 100,000 pascals), 14.73 psia, or 14.696 psia and the "standard" temperature is variously defined as 68 °F, 60 °F, 0 °C, 15 °C, 20 °C or 25 °C. The relative humidity (e.g., 36% or 0%) is also included in some definitions of standard conditions. There is, in fact, no universally accepted set of standard conditions. (See Standard conditions for temperature and pressure).
The temperature variation is the most important. In Europe, the standard temperature is most commonly defined as 0°C, but not always. In the United States, the standard temperature is most commonly defined as 60 °F or 70 °F, but again, not always. A variation in standard temperature can result in a significant volumetric variation for the same mass flow rate. For example, a mass flow rate of 1,000 kg/h of air at 1 atmosphere of absolute pressure is 455 SCFM when defined at 0 °C (32 °F) but 481 SCFM when defined at 60 °F (15.56 °C).
In countries using the SI metric system of unit, the term "normal cubic metre" (Nm3) is very often used to denote gas volumes at some normalized or standard condition. Again, as noted above, there is no universally accepted set of normalized or standard conditions.
Contents |
Actual cubic feet per minute
Actual cubic foot per minute (ACFM) is the volume of gas flowing anywhere in a system, independent of its temperature and pressure. If the system were moving a gas at exactly the "standard" condition, then ACFM would equal SCFM. Unfortunately, this usually is not the case as the most important change between these two definitions is the pressure. To move a gas, a positive pressure or a vacuum must be created. When positive pressure is applied to a standard cubic foot of gas, it is compressed. When a vacuum is applied to a standard cubic foot of gas, it expands. The volume of gas after it is pressurized or rarefied is referred to as its "actual" volume.
SCF and ACF for any gas are related in accordance with the combined gas law:[1][2][3]
Defining standard conditions by the subscript 1 and actual conditions by the subscript 2, then:[1][2][4]
where 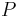 is in absolute pressure units and
is in absolute pressure units and 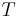 is in absolute temperature units (i.e., either kelvins or degrees Rankine).
is in absolute temperature units (i.e., either kelvins or degrees Rankine).
To be very precise when the gas is air, then the above equation should include correcting for the difference between the relative humidity of the air at the standard and the actual temperature and pressure conditions.[5] In most cases of engineering design, the humidity correction for air is often quite small and hence often ignored.
Cubic feet per minute
Cubic feet per minute (CFM) is an often confusing term because it has no single definition that applies to all instances. In the most basic sense, CFM means cubic feet per minute. Unfortunately, gases are compressible, which means that a figure in cubic feet per minute cannot be compared with another figure when it comes the mass of the gas. To further confuse the issue, a centrifugal fan is a constant CFM device or a constant volume device. This means that, provided the fan speed remains constant, a centrifugal fan will pump a constant volume of air. This is not the same as pumping a constant mass of air. Again, the fan will pump the same volume, though not mass, at any other air density. This means that the air velocity in a system is the same even though mass flow rate through the fan is not.
See also
- Gas laws
- Standard conditions for temperature and pressure
- Standard cubic foot (SCF)
- Million standard cubic feet per day (MMSCFD)
References
- ^ a b Controls Warehouse website (scroll down to "Gas Flow Measurement"
- ^ a b U.S. EPA website (scroll down to "Conversion between Actual and Standard Gas Flow Rates"
- ^ Mark Ladd (1998). Introduction to Physical Chemistry (3rd Edition ed.). Cambridge University Press. ISBN 0-521-57881-7. (Equation 5.2, page 200)
- ^ Robert J. Heinsohn and John M. Cimbala (2003). Indoor Air Quality Engineering: Environmental Health and Control of Indoor Pollutants. CRC Press. ISBN 0-8247-4061-0. (page 33)
- ^ SCFM versus ACFM (Specifically for air)
External links
- Xchanger Inc, webpage Calculator for SCFM, NM3/hr, lb/hr, kg/hr, ACFM & M3/hr gas flows.
- ACFM versus SCFM for ASME AG-1 HEPA Filters
- SCFM (Standard CFM) vs. ACFM (Actual CFM) (Specifically for air flows only)
- "Standard conditions for gases" from the IUPAC Gold Book.
- "Standard pressure" from the IUPAC Gold Book.
- "STP" from the IUPAC Gold Book.
- "Standard state" from the IUPAC Gold Book.
- Gas Density
- Properties of the Atmosphere