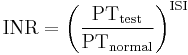Prothrombin time
| Prothrombin time | |
|---|---|
| Intervention | |
Blood plasma after the addition of Tissue Factor (Quick-Test). The gel-like structure is strong enough to hold a steel ball. |
|
| MeSH | D011517 |
The prothrombin time (PT) and its derived measures of prothrombin ratio (PR) and international normalized ratio (INR) are measures of the extrinsic pathway of coagulation. This test is also called "ProTime INR" and "INR PT". They are used to determine the clotting tendency of blood, in the measure of warfarin dosage, liver damage, and vitamin K status. PT measures factors I, II, V, VII, and X. It is used in conjunction with the activated partial thromboplastin time (aPTT) which measures the intrinsic pathway.
Contents |
Laboratory measurement
Normal range
The reference range for prothrombin time is usually around 10-13 seconds; the normal range for the INR is 0.8–1.2. Clinicians desiring therapeutic anticoagulation may aim for a higher INR - in many cases ranging from 2-3 - using anticoagulants such as warfarin.[1]
Methodology
The prothrombin time is most commonly measured using blood plasma. Blood is drawn into a test tube containing liquid citrate, which acts as an anticoagulant by binding the calcium in a sample. The blood is mixed, then centrifuged to separate blood cells from plasma. In newborns, a capillary whole blood specimen is used.[2]
The plasma is analyzed by a biomedical scientist on an automated instrument at 37°C, which takes a sample of the plasma. An excess of calcium is added (thereby reversing the effects of citrate), which enables the blood to clot again. For an accurate measurement the proportion of blood to citrate needs to be fixed; many laboratories will not perform the assay if the tube is underfilled and contains a relatively high concentration of citrate. If the tube is underfilled or overfilled with blood, the standardized dilution of 1 part anticoagulant to 9 parts whole blood is no longer valid. For the prothrombin time test the appropriate sample is the blue top tube, or sodium citrate tube, which is a liquid anticoagulant.
Tissue factor (also known as factor III) is added, and the time the sample takes to clot is measured optically. Some laboratories use a mechanical measurement, which eliminates interferences from lipemic and icteric samples. The prothrombin ratio is the prothrombin time for a patient, divided by the result for control plasma.
International normalised ratio
The result (in seconds) for a prothrombin time performed on a normal individual will vary according to the type of analytical system employed. This is due to the variations between different batches of manufacturer's tissue factor used in the reagent to perform the test. The INR was devised to standardize the results. Each manufacturer assigns an ISI value (International Sensitivity Index) for any tissue factor they manufacture. The ISI value indicates how a particular batch of tissue factor compares to an international reference tissue factor. The ISI is usually between 1.0 and 2.0. The INR is the ratio of a patient's prothrombin time to a normal (control) sample, raised to the power of the ISI value for the analytical system used.
Interpretation
The prothrombin time is the time it takes plasma to clot after addition of tissue factor (obtained from animals such as rabbits, or recombinant tissue factor, or from brains of autopsy patients). This measures the quality of the extrinsic pathway (as well as the common pathway) of coagulation. The speed of the extrinsic pathway is greatly affected by levels of functional factor VII in the body. Factor VII has a short half-life and the carboxylation of its glutamine residues requires vitamin K. The prothrombin time can be prolonged as a result of deficiencies in vitamin K, warfarin therapy, malabsorption, or lack of intestinal colonization by bacteria (such as in newborns). In addition, poor factor VII synthesis (due to liver disease) or increased consumption (in disseminated intravascular coagulation) may prolong the PT.
A high INR level such as INR=5 indicates that there is a high chance of bleeding, whereas if the INR=0.5 then there is a high chance of having a clot. Normal range for a healthy person is 0.9–1.3, and for people on warfarin therapy, 2.0–3.0, although the target INR may be higher in particular situations, such as for those with a mechanical heart valve, or bridging warfarin with a low-molecular weight heparin (such as enoxaparin) perioperatively.
| Condition | Prothrombin time | Partial thromboplastin time | Bleeding time | Platelet count |
|---|---|---|---|---|
| Vitamin K deficiency or warfarin | prolonged | normal or mildly prolonged | unaffected | unaffected |
| Disseminated intravascular coagulation | prolonged | prolonged | prolonged | decreased |
| von Willebrand disease | unaffected | prolonged | prolonged | unaffected |
| Hemophilia | unaffected | prolonged | unaffected | unaffected |
| Aspirin | unaffected | unaffected | prolonged | unaffected |
| Thrombocytopenia | unaffected | unaffected | prolonged | decreased |
| Liver failure, early | prolonged | unaffected | unaffected | unaffected |
| Liver failure, end-stage | prolonged | prolonged | prolonged | decreased |
| Uremia | unaffected | unaffected | prolonged | unaffected |
| Congenital afibrinogenemia | prolonged | prolonged | prolonged | unaffected |
| Factor V deficiency | prolonged | prolonged | unaffected | unaffected |
| Factor X deficiency as seen in amyloid purpura | prolonged | prolonged | unaffected | unaffected |
| Glanzmann's thrombasthenia | unaffected | unaffected | prolonged | unaffected |
| Bernard-Soulier syndrome | unaffected | unaffected | prolonged | decreased or unaffected |
Factors determining accuracy
Lupus anticoagulant, a circulating inhibitor predisposing for thrombosis, may skew PT results, depending on the assay used.[3] Variations between various thromboplastin preparations have in the past led to decreased accuracy of INR readings, and a 2005 study suggested that despite international calibration efforts (by INR) there were still statistically significant differences between various kits,[4] casting doubt on the long-term tenability of PT/INR as a measure for anticoagulant therapy.[5]
Statistics
An estimated 800 million PT/INR assays are performed annually worldwide.[5]
Near-patient testing
In addition to the laboratory method outlined above, near-patient testing (NPT) or home INR monitoring is becoming increasingly common in some countries. In the United Kingdom, for example, near-patient testing is used both by patients at home, and by some anticoagulation clinics (often hospital-based) as a fast and convenient alternative to the lab method. After a period of doubt about the accuracy of NPT results, a new generation of machines and reagents seems to be gaining acceptance for its ability to deliver results close in accuracy to those of the lab.[6]
In a typical NPT setup a small table-top device is used; for example the Roche Coaguchek S, the International Technidyne Corporation Hemochron Signature, or the more recently (2005) introduced HemoSense INRatio. A drop of capillary blood is obtained with an automated finger-prick, which is almost painless. This drop is placed on a disposable test strip with which the machine has been prepared. The resulting INR comes up on the display a few seconds later. Similar testing methods are used by diabetics on insulin, and are easily taught and practiced.
Local policy determines whether the patient or a coagulation specialist (pharmacist, nurse, general practitioner or hospital doctor) interprets the result and determines the dose of medication. In Germany, patients may adjust the medication dose themselves, while in the UK and the USA this remains in the hands of a health care professional. For example, patients using services such as Philips INR@Home [1] will phone in their INR results on a weekly basis and this information is transmitted to their doctor, who is also alerted if out-of-range levels should require an immediate intervention or adjustment to medications.
A significant advantage of home testing is the evidence that patient self-testing with medical support and patient self-management (where patients adjust their own anticoagulant dose) improves anticoagulant control. A meta analysis which reviewed 14 trials showed that home testing led to a reduced incidence of complications (bleeding and thrombosis) and improved the time in the therapeutic range, which is an indirect measure of anticoagulant control.[7]
Other advantages of the NPT approach are that it is fast and convenient, usually less painful, and offers, in home use, the ability for patients to measure their own INRs when required. Among its problems are that quite a steady hand is needed to deliver the blood to the exact spot, that some patients find the finger-pricking difficult, and that the cost of the test strips must also be taken into account. In the UK these are available on prescription so that elderly and unwaged people will not pay for them and others will pay only a standard prescription charge, which at the moment represents only about 20% of the retail price of the strips. In the USA, NPT in the home is currently reimbursed by Medicare for patients with mechanical heart valves, while private insurers may cover for other indications.Medicare is now covering home testing for patients with chronic atrial fibrillation. Home testing requires a doctor's prescription.
There is some evidence to suggest that NPT may be less accurate for certain patients, for example those who have the lupus anticoagulant.[8]
Guidelines
International guidelines were published in 2005 to govern home monitoring of oral anticoagulation by the International Self-Monitoring Association for Oral Anticoagulation.[9] The international guidelines study stated, “The consensus agrees that patient self-testing and patient self-management are effective methods of monitoring oral anticoagulation therapy, providing outcomes at least as good as, and possibly better than, those achieved with an anticoagulation clinic. All patients must be appropriately selected and trained. Currently available self-testing/self-management devices give INR results which are comparable with those obtained in laboratory testing.”
Medicare coverage for home testing of INR has been expanded in order to allow more people access to home testing of INR in the USA. The release on the 19th March 2008 said, “[t]he Centers for Medicare & Medicaid Services (CMS) expanded Medicare coverage for home blood testing of prothrombin time (PT) International Normalized Ratio (INR) to include beneficiaries who are using the drug warfarin, an anticoagulant (blood thinner) medication, for chronic atrial fibrillation or venous thromboembolism.” In addition, “[t]hose Medicare beneficiaries and their physicians managing conditions related to chronic atrial fibrillation or venous thromboembolism will benefit greatly through the use of the home test.”[10]
History
The prothrombin time was discovered by Dr Armand Quick and colleagues in 1935,[11] and a second method was published by Dr Paul Owren,[12] also called the "p and p" or "prothrombin and proconvertin" method. It aided in the identification of the anticoagulants dicumarol and warfarin,[13] and was used subsequently as a measure of activity for warfarin when used therapeutically.
The INR was introduced in the early 1980s when it turned out that there was a large degree of variation between the various prothrombin time assays, a discrepancy mainly due to problems with the purity of the thromboplastin (tissue factor) concentrate.[14] The INR became widely accepted worldwide, especially after endorsement by the World Health Organization.[15]
References
- ^ "Warfarin Therapy Management in Adults". http://www.bcguidelines.ca/gpac/pdf/warfarin_management_summary.pdf.
- ^ Fritsma, George A. "Evaluation of Hemostasis." Hematology: Clinical Principles and Applications . Ed. Bernadette Rodak. W.B. Saunders Company: Philadelphia, 2002. 719-53. Print
- ^ Della Valle P, Crippa L, Garlando AM, et al. (December 1999). "Interference of lupus anticoagulants in prothrombin time assays: implications for selection of adequate methods to optimize the management of thrombosis in the antiphospholipid-antibody syndrome" (PDF). Haematologica 84 (12): 1065–74. PMID 10586206. http://www.haematologica.org/cgi/reprint/84/12/1065.
- ^ Horsti J, Uppa H, Vilpo JA (March 2005). "Poor agreement among prothrombin time international normalized ratio methods: comparison of seven commercial reagents". Clin. Chem. 51 (3): 553–60. doi:10.1373/clinchem.2004.043836. PMID 15665046. http://www.clinchem.org/cgi/content/full/51/3/553.
- ^ a b Jackson CM, Esnouf MP (March 2005). "Has the time arrived to replace the quick prothrombin time test for monitoring oral anticoagulant therapy?". Clin. Chem. 51 (3): 483–5. doi:10.1373/clinchem.2004.045393. PMID 15738512. http://www.clinchem.org/cgi/content/full/51/3/483.
- ^ Poller L, Keown M, Chauhan N, et al. (September 2003). "European Concerted Action on Anticoagulation. Correction of displayed international normalized ratio on two point-of-care test whole-blood prothrombin time monitors (CoaguChek Mini and TAS PT-NC) by independent international sensitivity index calibration". Br. J. Haematol. 122 (6): 944–9. doi:10.1046/j.1365-2141.2003.04521.x. PMID 12956765.
- ^ Heneghan C, Alonso-Coello P, Garcia-Alamino JM, Perera R, Meats E, Glasziou P (February 2006). "Self-monitoring of oral anticoagulation: a systematic review and meta-analysis". Lancet 367 (9508): 404–11. doi:10.1016/S0140-6736(06)68139-7. PMID 16458764.
- ^ Moll, S and Ortel, TL. (August 1997). "Metering Warfarin Therapy in Patients with Lupus Anticoagulants.". Annals of Internal Medicine 127 (3): 177–185. PMID 9245222.
- ^ Jack Ansell (10 March 2005). "Guidelines for implementation of patient self-testing and patient self-management of oral anticoagulation. International consensus guidelines prepared by International Self-Monitoring Association for Oral Anticoagulation". International Journal of Cardiology. doi:10.1016/j.ijcard.2003.11.008. PMID 15721497. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T16-4CVR7GB-2&_user=10&_coverDate=03%2F10%2F2005&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=31d380c38e3d5afdba3f7dbb04e8b5b7.
- ^ "Medicare expands coverage for home blood testing of prothrombin time international normalized ratio". The Centers for Medicare and Medicaid Services. 19 March 2008. http://www.cms.hhs.gov/apps/media/press/release.asp?Counter=2987.
- ^ Quick AJ, Stanley-Brown M, Bancroft FW (1935). "A study of the coagulation defect in hemophilia and in jaundice". Am J Med Sci 190: 501. doi:10.1097/00000441-193510000-00009.
- ^ Owren PA, Aas K (1951). "The control of dicumarol therapy and the quantitative determination of prothrombin and proconvertin". Scand. J. Clin. Lab. Invest. 3 (3): 201–8. doi:10.3109/00365515109060600. PMID 14900966.
- ^ Campbell HA, Smith WK, Roberts WL, Link KP (1941). "Studies on the hemorrhagic sweet clover disease. II. The bioassay of hemorrhagic concentrates by following the prothrombin level in the plasma of rabbit blood". J Biol Chem 138: 1–20.
- ^ Hirsh J, Bates SM (March 2001). "Clinical trials that have influenced the treatment of venous thromboembolism: a historical perspective" (PDF). Ann. Intern. Med. 134 (5): 409–17. PMID 11242501. http://www.annals.org/cgi/content/full/134/5/409.
- ^ Anonymous (1983). "33: Expert Committee on Biological Standardization. Requirements for thromboplastins and plasma used to control oral anticoagulant therapy". World Health Organ Tech Rep Ser. pp. 81–105.
|
|||||||||||||||||||||