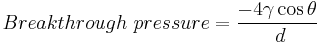Protection of exposed concrete
Protection of exposed concrete is necessary to prolong its service life. A 120 year design life for concrete infrastructure has become increasingly common. Without early preventive maintenance this design life target may be optimistic. The design service life of reinforced concrete structures often is not reached because of early deterioration and damage. The service life of some modern concrete structures is 20 to 30 years at most.[1] Even good quality engineering concrete is made up of countless invisible interconnecting capillary pores. The concrete acts like a “hard sponge” which absorbs damaging liquids such as water and aggressive water-borne salts. The strength of any concrete lessens as a result of static and dynamic loading, chloride ion ingress (from sea water) and the application of deicing salts. This leaves the concrete vulnerable to corrosion of the embedded steel. Concrete can also be damaged by sulfate attack, alkali silica reaction, carbonation, temperature change, abrasion, biological attack, salt crystallization, efflorescence and freeze-thaw attack.[2] Many of these processes are surface water driven and can be mitigated by early preventive maintenance with alkyl alkoxy silane impregnation.
Contents |
Capillary suction
Water and salts are drawn into the capillaries of the concrete via capillary suction and wicking, i.e. water moving from the saturated zone to the dry zone. The “war zones” for marine concrete are those areas that are subjected to the constant wet-dry cycles of wave action and wind. This causes oxygen, water and chloride ions to become plentiful. The corners of concrete in these areas are particularly vulnerable as they are attacked from two directions.
Capillary action is caused by surface tension and by the relative value of the adhesion between the water and the concrete to the cohesion of the water. The action of surface tension is to cause the water to rise within a small capillary that is partially immersed in the water. The water can rise to its maximum height occurs when the contact angle is zero. The smaller the pore radius the higher the water can rise. This is the same mechanism that gets water from the root of a tree to the tip of its highest leaf.
Chloride ion
Sea water contains approximately 3.5 wt. % salts by weight including; sodium chloride, magnesium sulphate, calcium sulphate and bicarbonates. In water these salts dissociate and migrate with the water into the capillaries of the concrete. Chloride ions are particularly aggressive for the corrosion of steel reinforcement bars and make up about 50 % of these ions. Chloride ions are easily incorporated in the crystal lattice of hydrated cement Afm phases where they form Friedel's salt. These salts can only penetrate into the concrete when dissolved in water. If the water is stopped from penetrating the concrete so are the salts it contains.
Saline water from the sea or de-icing salts (e.g. calcium chloride) provides a strong electrolyte to help drive the electro-potential corrosion of reinforcing steel. Local micro-environmental differences along the length of the reinforcement are enough to set up a potential difference and initiate corrosion. Chloride ions act a catalyst pulling the iron ions into solution to form rust in the presence of water and oxygen, resulting in the pitting corrosion of the steel. Rust can cause iron to expand 3-7 times its original size and this causes high tensile stress in surrounding concrete. If the stress is great enough the concrete will crack, spall and may delaminate the concrete cover.
De-icing salts such as calcium chloride and sodium chloride are added to road surfaces in winter to lower the freezing point of any surface water (over 50 million tons of de-icing salts are used each year on US roads). When it does freeze, unlike most liquids, water expands by 9 %. The cyclic freeze thaw action causes great pressures e.g. 200 MPa to build up in the pores causing micro-cracking and scaling.[3]
Sea water and some ground water contain many aggressive salts such as sodium chloride, calcium chloride, magnesium sulphate, sodium sulphate, calcium sulphate and bicarbonates. When these salts dry in the surface pores the resulting crystallization causes reduced cohesion of the cement paste, softens the concrete and reduces its strength.
Alkali silica reaction
When the alkalis in cement react with amorphous (i.e. non crystalline) silica aggregate in the presence of water then a disruptive swelling reaction occurs in the cement paste. When this happens to concrete railway sleepers the rails may end up out of alignment and cause a derailment.
Preventive treatment
Relative to the capillary pore size of concrete the alkyl alkoxy silane molecule is very small and can penetrate deeply. There are a number of silanes available and these vary with the alkyl part of the molecule to suit the application. For example some alkyl groups can only repel water but not oil and others can repel both water and oil. The preferred alkoxy group is ethoxy. The reason for this is because the ethoxy is a relatively slow reacting group allowing the silane to penetrate deeply, even displacing water that may be present, before reacting. Also, this group over time reacts with water in the pores or air to form ethanol which is a far safer and environmentally sound by-product than a methoxy group which would form methanol.
The result is a permanent connection with the capillary pore walls that repels liquids. The pores remain unblocked and allow the passage of water vapour, which being a gas has no surface tension. Allowing water vapour to escape has the effect of increasing the resistivity of the concrete and thus slowing down the rate of electro-potential corrosion of the reinforcing steel. Concrete can be silane-treated from the low tide level and above. It may take several treatments some weeks apart to obtain a good depth of impregnation between the low and high tide levels. Multiple applications over time allow the concrete to dry and allow for the silane to penetrate. Even though concrete with a pore diameter of say 2 × 10−6 m can temporarily resist over 4 m head of water pressure (P = -4x surface tension × Cos (contact angle) /pore diameter) i.e. only exposed surface should be treated . Below the low tide mark the available oxygen levels drops off steeply. At 15 °C at sea level there is only 7 mg of oxygen dissolved per litre of sea water. Air contains 250 mg of oxygen per litre, i.e. 36 time more oxygen available than in water.
When the capillaries are treated with silane the contact angle typically become 110 °. In the equation Cos (110 °) is a negative number and this describes the result of the treatment i.e. a repulsion of the water and salts. Only the pores are treated and there is no film or coating on the surface and so there is little or no change in its frictional properties or appearance.
Low Volatile Organic Compounds (VOCs) impregnating silane water-based creams are now available. VOCs include any volatile compound of carbon that participates in atmospheric photochemical reactions. In water repellents and related materials, a VOC is typically a formulation ingredient that will evaporate (volatilize) under normal use and is expressed as grams per liter. On July 1, 2006, the South Coast Air Quality Management District (SCAQMD) in California implemented the most stringent VOC requirements in the world. It is reasonable to expect that the rules we see in place in California today will influence a large portion of the world in the next few years. For SCAQMD, [waterproof|[waterproofing]] concrete sealers need to have VOC content below (excluding water and exempt compounds) 100 grams per Litre. The VOC content does not change regardless of how much water or exempt solvent is added to the sealer.[4]
Silane cream can be applied in one coat using a foam roller or low pressure spray unit. The typical application rate is 1 litre per 3 to 4 sq.m. depending on the surface absorption and depth of penetration required. It needs to be protected from moisture for a minimum of 12 hours. The 21 day reduction in 15 % NaCl brine water uptake in accordance NCHRP Report 244 method is up to 70 % for liquid silane and 64 % for cream.[5]
The cream combines the best aspects of both water-based and solvent-based technologies. The cream sits on the surface and once the water evaporates the solvents take over to penetrate deeply into the surface. Unlike a typical water-based coating the cream can even pass through a previously impregnated (i.e. water repelling surface). The cream is applied in one coat and allows the applicator better control particularly in windy conditions and on vertical and overhead surfaces.
If not used correctly, solvent-based silanes pose significant health and safety problems with potential risk of skin and eye irritation and damage to vegetation and aquatic environments. There is little risk in using cream-based products.[6]
Impregnation undertaken in the UK is known to be effective for at least 15 years, provided it is applied correctly. Longer service life is anticipated.[7]
Service life prediction
With construction standards in the United States and several other countries turning towards performance-based requirements, concrete service life prediction has grown in importance. Service life modelling software (such as STADIUM) is now recommended by the US Navy for new waterfront construction.[8]
Case study
The quay-wall of a new container terminal at Zeebrugge Harbor, Belgium, was protected against chloride ingress by means of a water-repellent agent immediately after construction in 1993. An alkyl triethoxy silane was used pre-evaluated in a preliminary research program. To judge the in site effectiveness of the hydrophobic agent as a water repellent treatment, three subsequent in site surveys were conducted in 1996, 1998 and recently in 2005. Based on the cores drilled, the chloride profiles are determined as a function of time, both in a non-treated and treated location. Because of the long-term data sequence, the long-term effectiveness of the treatment can be assessed in an objective way. Predicted service life represents the 50 % probability of chloride ions to reaching reinforcing bars at a depth of 120 mm with a sufficient concentration of 0.7 % chloride ion by weight of cement to start the corrosion process i.e. untreated 16.5 years, treated 107 years.[9]
Depth of impregnation
Amongst the most decisive parameters determining the effectiveness of a water repellent treatment is the penetration depth. A good depth of penetration not only protects the treatment from weathering and traffic but also sets up a hydrophobic barrier. The depth of penetration is tested by breaking open the treated specimen and spraying the fractured surface with water. The depth of the dry zone is taken as the effective depth of impregnation. This barrier stops the passage of liquid water and salts into or out of the concrete. Depth of penetration is also a good quality control check. A 50 mm diameter 40 mm deep button core is recommended to be randomly taken for every 300 sq.m. of concrete treated. This can be compared with a known sample to ensure that the contractor is applying the correct quantity of silane. It should be greater than or equal to 5 mm. It should be noted that existing treated surfaces can easily absorb more applications and the new material will penetrate deeper levels in the concrete. This is enhanced by waiting for each application to fully dry.
Alberta Transportation and Utilities[10] carried out a series of five day water immersion tests to measure the reduction in water uptake on concrete samples treated with common sealers i.e. epoxy, acrylic, siloxane and silane. The water uptake was also measured after sandblasting to abrade (total of 3 % removed by weight) the surface as a means of simulated accelerated weathering. Only the silanes kept on performing after significant surface abrasion. It is estimated that a highway concrete wearing surface will lose 1 mm of concrete every 7 years.
Theoretical break through pressure
The anti-capillary force caused by the silane treatment can be overcome by applying a sufficiently high force such as extreme wind driven rain pressure or by hydrostatic pressure on the liquid trying to enter the pore. The amount of force needed to push the water into the concrete is proportional to the imparted critical surface tension of the substrate and the pore’s diameter.
where:
 : the liquid-air surface tension (energy/surface area)
: the liquid-air surface tension (energy/surface area)- θ: the contact angle (angle)
- d: diameter of pore (length)
For a concrete, using SI units:
 = 0.072 J/m2 at 20 °C
= 0.072 J/m2 at 20 °C- θ = 107 ° (angle)
- d = 2.1 × 10−6 m
Breakthrough pressure is 40,100 N/m2 or 4.092 metres (i.e. 40100/101325 × 10.34 = 4.092 m) pressure head of water. By comparison 50 mm pressure head is the same pressure from 104 km per hour wind driven rain.
If the pore size increases, the head pressure resistance will decrease. Water vapour does not have a surface tension, it can pass freely through a substrate made water repellent with the silane treatment.
Conclusion
Many of the processes that deteriorate the strength of engineering concrete are surface-water driven and can be mitigated by early preventive maintenance with an alkyl alkoxy impregnating silane. The silane is used to line capillary pores and make them hydrophobic stopping capillary suction and wicking action These products are non-film forming; able to greatly reduce water uptake; an excellent chloride ion screen; highly water vapour permeable; deeply penetrating; very alkali resistant; do not change the appearance or frictional property of the surface and can seal hairline cracks.
References
- ^ Penetration depth of different water repellent agents, by Zhan, Wittman and Zhau (2003)
- ^ Vassie pp. 86 Long-term maintenance strategies for highway bridges Bridge Engineering 159 issue BE2
- ^ Dr. Zhou, Achieving durability in design process In-ground structures pp. 7.
- ^ David Selley (2007) Q&A: Understanding VOCs for water-repellent materials for concrete, Dow Corning Corporation, pp. 15–20 JPCL, June 2007.
- ^ Wiss Janney Elstner Associates, Testing of concreme N° 2009.0361
- ^ TRL Limited A. Calder and M. McKenzie "Performance of impregnants" October 2009.
- ^ The impregnation of reinforced and prestressed concrete highway structures using hydrophobic pore-lining impregnants. UK design manual for roads and bridges DB43/03.
- ^ United Facilities Guide Specifications (UFGS-03 31 29), February 2010, http://www.wbdg.org/ccb/DOD/UFGS/UFGS%2003%2031%2029.pdf
- ^ L. Schueremans et al., Durability of hydrophobic agents applied in a marine environment. In: 5th International Conference on Water Repellent Treatment of Building Materials. Aedificatio Publishers, 1- 11 (2007).
- ^ Evaluation of damproofing performance and effective penetration depth of silane sealers in concrete, November 1993.