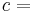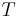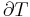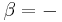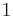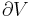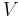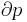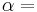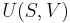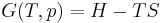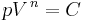Polytropic process
A polytropic process is a thermodynamic process that obeys the relation:
where p is the pressure, V is volume, n, the polytropic index, is any real number, and C is a constant. This equation can be used to accurately characterize processes of certain systems, notably the compression or expansion of a gas and in some cases liquids and solids.
Contents |
Applicability
The equation is a valid characterization of a thermodynamic process assuming that the process is quasistatic and the values of the heat capacities, 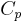 and
and 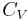 , are almost constant when
, are almost constant when 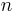 is not zero or infinity. (In reality,
is not zero or infinity. (In reality, 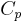 and
and 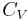 are actually functions of temperature and pressure, but are nearly constant within small changes of temperature).
are actually functions of temperature and pressure, but are nearly constant within small changes of temperature).
Under standard conditions, most gases can be accurately characterized by the ideal gas law. This construct allows for the pressure-volume relationship to be defined for essentially all ideal thermodynamic cycles, such as the well-known Carnot cycle. Note however that there may also be instances where a polytropic process occurs in a non-ideal gas.
Relationship to ideal processes
For certain values of the polytropic index, the process will be synonymous with other common processes. Some examples of the effects of varying index values are given in the table.
| Polytropic index |
Relation | Effects |
|---|---|---|
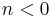 |
— | An explosion occurs |
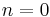 |
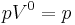 (constant) |
Equivalent to an isobaric process (constant pressure) |
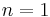 |
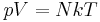 (constant) |
Equivalent to an isothermal process (constant temperature) |
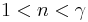 |
— | A quasi-adiabatic process such as in an internal combustion engine during expansion, or in vapor compression refrigeration during compression |
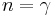 |
— | 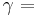 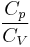 is the adiabatic index, yielding an adiabatic process (no heat transferred) is the adiabatic index, yielding an adiabatic process (no heat transferred) |
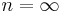 |
— | Equivalent to an isochoric process (constant volume) |
When the index n is between any two of the former values (0, 1, gamma, or infinity), it means that the polytropic curve will bounded by the curves of the two corresponding indices.
Note that 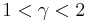 , since
, since 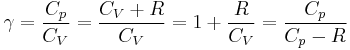 .
.
Notation
In the case of an isentropic ideal gas, 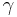 is the ratio of specific heats, known as the adiabatic index or as adiabatic exponent.
is the ratio of specific heats, known as the adiabatic index or as adiabatic exponent.
An isothermal ideal gas is also a polytropic gas. Here, the polytropic index is equal to one, and differs from the adiabatic index 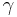 .
.
In order to discriminate between the two gammas, the polytropic gamma is sometimes capitalized, 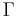 .
.
To confuse matters further, some authors refer to 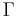 as the polytropic index, rather than
as the polytropic index, rather than 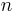 . Note that
. Note that
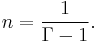
Other
A solution to the Lane-Emden equation using a polytropic fluid is known as a polytrope.