Spin isomers of hydrogen
Molecular hydrogen occurs in two isomeric forms, one with its two proton spins aligned parallel (orthohydrogen), the other with its two proton spins aligned antiparallel (parahydrogen).[1] At room temperature and thermal equilibrium, hydrogen consists of 25% parahydrogen and 75% orthohydrogen.
Nuclear spin states of H2
Each hydrogen molecule (H2) consists of two hydrogen atoms linked by a covalent bond. If we neglect the small proportion of deuterium and tritium which may be present, each hydrogen atom consists of one proton and one electron. The proton has an associated magnetic moment, which is associated with the proton's spin. In the H2 molecule, the spins of the two hydrogen nuclei (protons) couple to form a triplet state (I = 1, α1α2, (α1β2 + β1α2)/(21/2), or β1β2 for which MI = 1, 0, −1 respectively — this is orthohydrogen) or to form a singlet state (I = 0, (α1β2 – β1α2)/(21/2) MI = 0 — this is parahydrogen). The ratio between the ortho and para forms is about 3:1 at standard temperature and pressure - a reflection of the spin degeneracy ratio, but if thermal equilibrium between the two forms is established, the para form dominates at low temperatures (approx. 99.8% at 20 K[2]). Other molecules and functional groups containing two hydrogen atoms, such as water and methylene, also have ortho and para forms (e.g. orthowater and parawater), although their ratios differ from that of the dihydrogen molecule.
Thermal properties
The permutational antisymmetry of the H2 wavefunction (protons are fermions) imposes restrictions on the possible rotational states the two forms of H2 can adopt. Orthohydrogen, with symmetric nuclear spin functions, can only have rotational wavefunctions that are antisymmetric with respect to permutation of the two protons. Conversely, parahydrogen with an antisymmetric nuclear spin function, can only have rotational wavefunctions that are symmetric with respect to permutation of the two protons. Applying the rigid rotor approximation, the energies and degeneracies of the rotational states are given by[3]
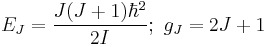 .
.
The rotational partition function is conventionally written as
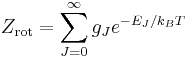 .
.
However, as long as these two spin isomers are not in equilibrium, it is more useful to write separate partition functions for each,
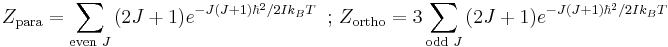 .
.
The factor of 3 in the partition function for orthohydrogen accounts for the spin degeneracy associated with the +1 spin state. When equilibrium between the spin isomers is possible, then a general partition function incorporating this degeneracy difference can be written as
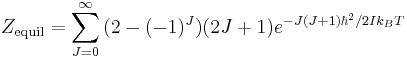
The molar rotational energies and heat capacities are derived for any of these cases from
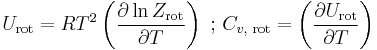
Because of the antisymmetry-imposed restriction on possible rotational states, orthohydrogen has residual rotational energy at low temperature wherein nearly all the molecules are in the J = 1 state (molecules in the symmetric spin-triplet state cannot fall into the lowest, symmetric rotational state) and possesses nuclear-spin entropy due to the triplet state's threefold degeneracy. The residual energy is significant because the rotational energy levels are relatively widely spaced in H2; the gap between the first two levels when expressed in temperature units is twice the rotational temperature for H2,
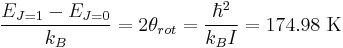 .
.
This is the T = 0 intercept seen in the molar energy of orthohydrogen. This residual energy, 1091 J/mol, is somewhat larger than the enthalpy of vaporization of normal hydrogen, 904 J/mol at the boiling point, Tb = 20.369 K (this refers to the "normal", room-temperature, 3:1 ortho:para mixture).[1] Notably, the boiling points of parahydrogen and normal (3:1) hydrogen are nearly equal; for parahydrogen ∆Hvap = 898 J/mol at Tb = 20.277 K. It follows that nearly all the residual rotational energy of orthohydrogen is retained in the liquid state. Orthohydrogen is consequently unstable at low temperatures and spontaneously converts into parahydrogen, but the process is slow in the absence of a magnetic catalyst to facilitate interconversion of the singlet and triplet spin states. At room temperature, hydrogen contains 75% orthohydrogen, a proportion which the liquefaction process preserves if carried out in the absence of a catalyst like ferric oxide, activated carbon, platinized asbestos, rare earth metals, uranium compounds, chromic oxide, or some nickel compounds[4] to accelerate the conversion of the liquid hydrogen into parahydrogen, or supply additional refrigeration equipment to absorb the heat that the orthohydrogen fraction will release as it spontaneously converts into parahydrogen. If orthohydrogen is not removed from liquid hydrogen, the heat released during its decay can boil off as much as 50% of the original liquid[5].
The first synthesis of pure parahydrogen was achieved by Paul Harteck and Karl Friedrich Bonhoeffer in 1929.
Modern isolation of pure parahydrogen has been achieved utilizing rapid in-vacuum deposition of millimeters thick solid parahydrogen (pH2) samples which are notable for their excellent optical qualities.[6]
Further research regarding parahydrogen thinfilm quantum state polarization matrices for computation seems a likely future prospect for these material sets.
When an excess of parahydrogen is used during hydrogenation reactions, the resultant product exhibits hyperpolarized signals in proton NMR spectra. This effect is called Parahydrogen Inducded Polarisation (PHIP) and has utilized to study the mechanism of hydrogenation reactions.
References
- ^ P. Atkins and J. de Paula, Atkins' Physical Chemistry, 8th edition (W.H.Freeman 2006), p.452
- ^ Rock, Peter A. "Chemical Thermodynamics", MacMillan 1969, p.478
- ^ F. T. Wall (1974). Chemical Thermodynamics, 3rd Edition. W. H. Freeman and Company.
- ^ Ortho-Para conversion. Pag. 13
- ^ http://www.mae.ufl.edu/NasaHydrogenResearch/h2webcourse/L11-liquefaction2.pdf
- ^ Rapid Vapor Deposition of Millimeters Thick Optically Transparent Solid Parahydrogen Samples for Matrix Isolation Spectroscopy
- Tikhonov V. I., Volkov A. A. (2002). "Separation of water into its ortho and para isomers". Science 296 (5577): 2363. doi:10.1126/science.1069513. PMID 12089435.
- Bonhoeffer KF, Harteck P (1929). "Para- and ortho hydrogen". Zeitschrift für Physikalische Chemie B 4 (1-2): 113–141.
- A. Farkas (1935). Orthohydrogen, parahydrogen and heavy hydrogen,. The Cambridge series of physical chemistry.
- Mario E. Fajardo; Simon Tam (1997). Rapid Vapor Deposition of Millimeters Thick Optically Transparent Solid Parahydrogen Samples for Matrix Isolation Spectroscopy. AIR FORCE RESEARCH LAB EDWARDS AFB CA PROPULSION DIRECTORATE WEST.