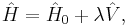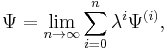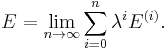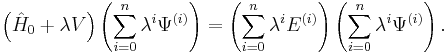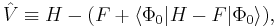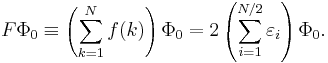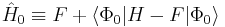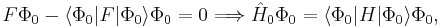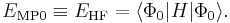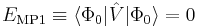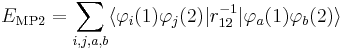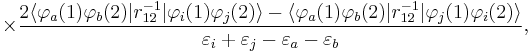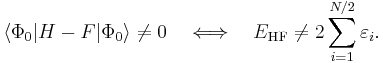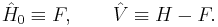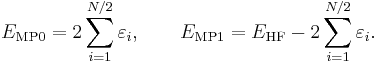Møller–Plesset perturbation theory
Møller–Plesset perturbation theory (MP) is one of several quantum chemistry post-Hartree–Fock ab initio methods in the field of computational chemistry. It improves on the Hartree–Fock method by adding electron correlation effects by means of Rayleigh–Schrödinger perturbation theory (RS-PT), usually to second (MP2), third (MP3) or fourth (MP4) order. Its main idea was published as early as 1934 by Christian Møller and Milton S. Plesset.[1]
Contents |
Rayleigh–Schrödinger perturbation theory
The MP-theory is a special application of RS-PT. In RS-PT one considers an unperturbed Hamiltonian operator 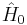 to which is added a small (often external) perturbation
to which is added a small (often external) perturbation 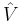 :
:
where λ is an arbitrary real parameter. In MP theory the zeroth-order wave function is an exact eigenfunction of the Fock operator, which thus serves as the unperturbed operator. The perturbation is the correlation potential.
In RS-PT the perturbed wave function and perturbed energy are expressed as a power series in λ:
Substitution of these series into the time-independent Schrödinger equation gives a new equation: (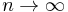 )
)
Equating the factors of 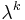 in this equation gives a kth-order perturbation equation, where k = 0, 1, 2, ..., n. See perturbation theory for more details.
in this equation gives a kth-order perturbation equation, where k = 0, 1, 2, ..., n. See perturbation theory for more details.
Møller–Plesset perturbation
Original formulation
The MP-energy corrections are obtained from Rayleigh–Schrödinger (RS) perturbation theory with the perturbation (correlation potential):
where the normalized Slater determinant Φ0 is the lowest eigenfunction of the Fock operator
Here N is the number of electrons of the molecule under consideration, H is the usual electronic Hamiltonian, 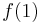 is the one-electron Fock operator, and εi is the orbital energy belonging to the doubly occupied spatial orbital φi. The shifted Fock operator
is the one-electron Fock operator, and εi is the orbital energy belonging to the doubly occupied spatial orbital φi. The shifted Fock operator
serves as the unperturbed (zeroth-order) operator.
The Slater determinant Φ0 being an eigenfunction of F, it follows readily that
so that the zeroth-order energy is the expectation value of H with respect to Φ0, i.e., the Hartree–Fock energy:
Since the first-order MP energy
is obviously zero, the lowest-order MP correlation energy appears in second order. This result is the Møller–Plesset theorem:[1] the correlation potential does not contribute in first-order to the exact electronic energy.
In order to obtain the MP2 formula for a closed-shell molecule, the second order RS-PT formula is written on basis of doubly excited Slater determinants. (Singly excited Slater determinants do not contribute because of the Brillouin theorem). After application of the Slater–Condon rules for the simplification of N-electron matrix elements with Slater determinants in bra and ket and integrating out spin, it becomes
where φi and φj are canonical occupied orbitals and φa and φb are canonical virtual orbitals. The quantities εi, εj, εa, and εb are the corresponding orbital energies. Clearly, through second-order in the correlation potential, the total electronic energy is given by the Hartree–Fock energy plus second-order MP correction: E ≈ EHF + EMP2. The solution of the zeroth-order MP equation (which by definition is the Hartree–Fock equation) gives the Hartree–Fock energy. The first non-vanishing perturbation correction beyond the Hartree–Fock treatment is the second-order energy.
Alternative formulation
Equivalent expressions are obtained by a slightly different partitioning of the Hamiltonian, which results in a different division of energy terms over zeroth- and first-order contributions, while for second- and higher-order energy corrections the two partitionings give identical results. The formulation is commonly used by chemists, who are now large users of these methods.[2] This difference is due to the fact, well-known in Hartree–Fock theory, that
(The Hartree–Fock energy is not equal to the sum of occupied-orbital energies). In the alternative partitioning, one defines
Clearly in this partitioning,
Obviously, the Møller–Plesset theorem does not hold in the sense that EMP1 ≠ 0. The solution of the zeroth-order MP equation is the sum of orbital energies. The zeroth plus first-order correction yields the Hartree–Fock energy. As with the original formulation, the first non-vanishing perturbation correction beyond the Hartree–Fock treatment is the second-order energy. We reiterate that the second- and higher-order corrections are the same in both formulations.
Use of Møller–Plesset perturbation methods
Second (MP2),[3] third (MP3),[4][5] and fourth (MP4)[6] order Møller–Plesset calculations are standard levels used in calculating small systems and are implemented in many computational chemistry codes. Higher level MP calculations, generally only MP5,[7] are possible in some codes. However, they are rarely used because of their cost.
Systematic studies of MP perturbation theory have shown that it is not necessarily a convergent theory at high orders. Convergence can be slow, rapid, oscillatory, regular, highly erratic or simply non-existent, depending on the precise chemical system or basis set.[8] The density matrix for the first-order and higher MP2 wavefunction is of the type known as response density, which differs from the more usual expectation value density.[9][10] The eigenvalues of the response density matrix (which are the occupation numbers of the MP2 natural orbitals) can therefore be greater than 2 or negative. Unphysical numbers are a sign of a divergent perturbation expansion.[11]
Additionally, various important molecular properties calculated at MP3 and MP4 level are no better than their MP2 counterparts, even for small molecules.[12]
For open shell molecules, MPn-theory can directly be applied only to unrestricted Hartree–Fock reference functions (since ROHF states are not in general eigenvectors of the Fock operator). However, the resulting energies often suffer from severe spin contamination, leading to large errors. A possible better alternative is to use one of the MP2-like methods based on restricted open-shell Hartree–Fock (ROHF). Unfortunately, there are many ROHF based MP2-like methods because of arbitrariness in the ROHF wavefunction[13][14](for example HCPT,[15] ROMP,[16] RMP[17] (also called ROHF-MBPT2[18]), OPT1 and OPT2,[19] ZAPT,[20] IOPT,[21] etc.[22][23]). Some of the ROHF based MP2-like theories suffer from spin-contamination in their perturbed density and energies beyond second-order.
These methods, Hartree–Fock, unrestricted Hartree–Fock and restricted Hartree–Fock use a single determinant wave function. Multi-configurational self-consistent field (MCSCF) methods use several determinants and can be used for the unperturbed operator, although not uniquely, so many methods, such as complete active space perturbation theory (CASPT2),[24] and Multi-Configuration Quasi-Degenerate Perturbation Theory (MCQDPT),[25][26] have been developed.[27] Unfortunately, MCSCF based methods are not without perturbation series divergences.[28]
See also
- Electron correlation
- Perturbation theory (quantum mechanics)
- Post-Hartree–Fock
- List of quantum chemistry and solid state physics software
References
- ^ a b Møller, Christian; Plesset, Milton S. (1934). "Note on an Approximation Treatment for Many-Electron Systems" (abstract). Phys. Rev. 46 (7): 618–622. Bibcode 1934PhRv...46..618M. doi:10.1103/PhysRev.46.618. http://link.aps.org/abstract/PR/v46/p618.
- ^ See all volumes in the "Further reading" section.
- ^ Head-Gordon, Martin; Pople, John A.; Frisch, Michael J. (1988). "MP2 energy evaluation by direct methods". Chemical Physics Letters 153 (6): 503–506. Bibcode 1988CPL...153..503H. doi:10.1016/0009-2614(88)85250-3.
- ^ Pople, J. A.; Seeger, R.; Krishnan, R. (1977). "Variational configuration interaction methods and comparison with perturbation theory" (abstract). International Journal of Quantum Chemistry 12 (S11): 149–163. doi:10.1002/qua.560120820. http://www3.interscience.wiley.com/journal/122460463/abstract.
- ^ Pople, John A.; Binkley, J. Stephen; Seeger, Rolf (1976). "Theoretical models incorporating electron correlation" (abstract). International Journal of Quantum Chemistry 10 (S10): 1–19. doi:10.1002/qua.560100802. http://www3.interscience.wiley.com/journal/122460410/abstract.
- ^ Krishnan, Raghavachari; Pople, John A. (1978). "Approximate fourth-order perturbation theory of the electron correlation energy". International Journal of Quantum Chemistry 14 (1): 91–100. doi:10.1002/qua.560140109.
- ^ Raghavachari, Krishnan.; Pople, John A.; Replogle, Eric S.; Head-Gordon, Martin (1990). "Fifth order Moeller-Plesset perturbation theory: comparison of existing correlation methods and implementation of new methods correct to fifth order". The Journal of Physical Chemistry 94 (14): 5579–5586. doi:10.1021/j100377a033.
- ^ Leininger, Matthew L.; Allen, Wesley D.; Schaeferd, Henry F.; Sherrill, C. David (2000). "Is Moller–Plesset perturbation theory a convergent ab initio method?". J. Chem. Phys. 112 (21): 9213–9222. Bibcode 2000JChPh.112.9213L. doi:10.1063/1.481764.
- ^ Handy, Nicholas C.; Schaefer, Henry F. (1984). "On the evaluation of analytic energy derivatives for correlated wave functions". The Journal of Chemical Physics 81 (11): 5031. Bibcode 1984JChPh..81.5031H. doi:10.1063/1.447489.
- ^ Wiberg, Kenneth B.; Hadad, Christopher M.; Lepage, Teresa J.; Breneman, Curt M.; Frisch, Michael J. (1992). "Analysis of the effect of electron correlation on charge density distributions". The Journal of Physical Chemistry 96 (2): 671. doi:10.1021/j100181a030.
- ^ Gordon, Mark S.; Schmidt, Michael W.; Chaban, Galina M.; Glaesemann, Kurt R.; Stevens, Walter J.; Gonzalez, Carlos (1999). "A natural orbital diagnostic for multiconfigurational character in correlated wave functions". J. Chem. Phys. 110 (9): 4199–4207. Bibcode 1999JChPh.110.4199G. doi:10.1063/1.478301.
- ^ Helgaker, Trygve; Poul Jorgensen and Jeppe Olsen (2000). Molecular Electronic Structure Theory. Wiley. ISBN 978-0471967552.
- ^ Glaesemann, Kurt R.; Schmidt, Michael W. (2010). "On the Ordering of Orbital Energies in High-Spin ROHF†". The Journal of Physical Chemistry A 114 (33): 8772–8777. doi:10.1021/jp101758y. PMID 20443582.
- ^ Crawford, T. Daniel; Schaefer, Henry F.; Lee, Timothy J. (1996). "On the energy invariance of open-shell perturbation theory with respect to unitary transformations of molecular orbitals". The Journal of Chemical Physics 105 (3): 1060. Bibcode 1996JChPh.105.1060C. doi:10.1063/1.471951.
- ^ Hubač, Ivan; Čársky, Petr (1980). "Correlation energy of open-shell systems. Application of the many-body Rayleigh-Schrödinger perturbation theory in the restricted Roothaan-Hartree-Fock formalism". Physical Review A 22 (6): 2392–2399. Bibcode 1980PhRvA..22.2392H. doi:10.1103/PhysRevA.22.2392.
- ^ Amos, Roger D.; Andrews, Jamie S.; Handy, Nicholas C.; Knowles, Peter J. (1991). "Open-shell Møller—Plesset perturbation theory". Chemical Physics Letters 185 (3–4): 256–264. Bibcode 1991CPL...185..256A. doi:10.1016/S0009-2614(91)85057-4.
- ^ Knowles, Peter J.; Andrews, Jamie S.; Amos, Roger D.; Handy, Nicholas C.; Pople, John A. (1991). "Restricted Møller—Plesset theory for open-shell molecules". Chemical Physics Letters 186 (2–3): 130–136. Bibcode 1991CPL...186..130K. doi:10.1016/S0009-2614(91)85118-G.
- ^ Lauderdale, Walter J.; Stanton, John F.; Gauss, Jürgen; Watts, John D.; Bartlett, Rodney J. (1991). "Many-body perturbation theory with a restricted open-shell Hartree—Fock reference". Chemical Physics Letters 187 (1–2): 21–28. Bibcode 1991CPL...187...21L. doi:10.1016/0009-2614(91)90478-R.
- ^ Murray, Christopher; Davidson, Ernest R. (1991). "Perturbation theory for open shell systems". Chemical Physics Letters 187 (5): 451–454. Bibcode 1991CPL...187..451M. doi:10.1016/0009-2614(91)80281-2.
- ^ Lee, Timothy J.; Jayatilaka, Dylan (1993). "An open-shell restricted Hartree—Fock perturbation theory based on symmetric spin orbitals". Chemical Physics Letters 201 (1–4): 1–10. Bibcode 1993CPL...201....1L. doi:10.1016/0009-2614(93)85024-I.
- ^ Kozlowski, P. M.; Davidson, Ernest R. (1994). "Construction of open shell perturbation theory invariant with respect to orbital degeneracy". Chemical Physics Letters 226 (5–6): 440–446. Bibcode 1994CPL...226..440K. doi:10.1016/0009-2614(94)00763-2.
- ^ Murray, Christopher W.; Handy, Nicholas C. (1992). "Comparison and assessment of different forms of open shell perturbation theory". The Journal of Chemical Physics 97 (9): 6509. Bibcode 1992JChPh..97.6509M. doi:10.1063/1.463680.
- ^ Murray, Christopher; Davidson, Ernest R. (1992). "Different forms of perturbation theory for the calculation of the correlation energy". International Journal of Quantum Chemistry 43 (6): 755. doi:10.1002/qua.560430604.
- ^ Roos, Bjrn O; Andersson, Kerstin; Flscher, Markus P; Malmqvist, Per-ke; Serrano-Andrs, Luis; Pierloot, Kristin; Merchn, Manuela (1996). Multiconfigurational Perturbation Theory: Applications in Electronic Spectroscopy. 93. pp. 219. doi:10.1002/9780470141526.ch5.
- ^ Nakano, Haruyuki (1993). "Quasidegenerate perturbation theory with multiconfigurational self-consistent-field reference functions". The Journal of Chemical Physics 99 (10): 7983. Bibcode 1993JChPh..99.7983N. doi:10.1063/1.465674.
- ^ Granovsky, A. A. (2011). "Extended multi-configuration quasi-degenerate perturbation theory: The new approach to multi-state multi-reference perturbation theory". J. Chem. Phys. 134 (21): 214113. Bibcode 2011JChPh.134u4113G. doi:10.1063/1.3596699.
- ^ Davidson, Ernest R.; Jarzecki, A. A. (1999). K. Hirao. ed. Recent Advances in Multireference Methods. World Scientific. pp. 31–63. ISBN 981-02-3777-4.
- ^ Glaesemann, Kurt R.; Gordon, Mark S.; Nakano, Haruyuki (1999). "A study of FeCO+ with correlated wavefunctions". Physical Chemistry Chemical Physics 1 (6): 967–975. Bibcode 1999PCCP....1..967G. doi:10.1039/a808518h.
Further reading
- Cramer, Christopher J. (2002). Essentials of Computational Chemistry. Chichester: John Wiley & Sons, Ltd.. pp. 207–211. ISBN 0-471-48552-7.
- Foresman, James B.; Æleen Frisch (1996). Exploring Chemistry with Electronic Structure Methods. Pittsburgh, PA: Gaussian Inc.. pp. 267–271. ISBN 0-9636769-4-6.
- Leach, Andrew R. (1996). Molecular Modelling. Harlow: Longman. pp. 83–85. ISBN 0-582-23933-8.
- Levine, Ira N. (1991). Quantum Chemistry. Englewood Cliffs, New jersey: Prentice Hall. pp. 511–515. ISBN 0-205-12770-3.
- Szabo, Attila; Neil S. Ostlund (1996). Modern Quantum Chemistry. Mineola, New York: Dover Publications, Inc. pp. 350–353. ISBN 0-486-69186-1.