Mannose
| Mannose | |
|---|---|
D-Mannopyranose
|
|
Fischer projections
|
|
| Identifiers | |
| CAS number | 31103-86-3 |
| PubChem | 18950 |
| UNII | PHA4727WTP |
| MeSH | Mannose |
| ChEMBL | CHEMBL469448 |
| Properties | |
| Molecular formula | C6H12O6 |
| Molar mass | 180.16 g mol−1 |
| (verify) (what is: /?) Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Mannose is a sugar monomer of the aldohexose series of carbohydrates. Mannose is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism.[1]
Contents |
Structure
Two of the cyclic mannose isomers possess a pyranose (six-membered) ring, while the other two possess a furanose (five-membered) ring.
| D-Mannose isomers | |
|---|---|
| Haworth projections | |
α-D-Mannofuranose <1 % |
β-D-Mannofuranose <1 % |
α-D-Mannopyranose 67 % |
β-D-Mannopyranose 33 % |
Metabolism
While much of the mannose used in glycosylation is believed to be derived from glucose, in cultured hepatoma (liver-derived) cells, most of the mannose for glycoprotein biosynthesis comes from extracellular mannose, not glucose. [2] Many of the glycoproteins produced in the liver are secreted into the bloodstream, so dietary mannose is distributed throughout the body. [3]
Mannose is present in numerous glycoconjugates including N-linked glycosylation of proteins. C-mannosylation is also abundant and can be found in collagen-like regions.
Biotechnology
Recombinant proteins produced in yeast may be subject to mannose addition in patterns different from those used by mammalian cells.[4] This difference in recombinant proteins from those normally produced in mammalian organisms may influence the effectiveness of vaccines.
Formation
Mannose can be formed by the oxidation of mannitol.
It can also be formed from glucose in the Lobry-de Bruyn-van Ekenstein transformation
Therapeutic uses
D-mannose is sold as a naturopathic remedy for urinary tract infections, and it is claimed to work through the disruption of adherence of bacteria in the urinary tract.
Etymology
The root of both "mannose" and "mannitol" is manna, which the Bible records as the food supplied to the Israelites during their journey through the Sinai Peninsula. Manna is a sweet secretion of several trees and shrubs, such as Fraxinus ornus.
Configuration
Mannose differs from glucose by inversion of the C-2 chiral center. Mannose displays a 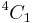 pucker in the solution ring form.
pucker in the solution ring form.
This apparently simple change leads to the drastically different chemistry of the two hexoses, as it does the remaining six aldohexoses.
See also
References
- ^ Metabolic Manipulation of Glycosylation Disorders in Humans and Animal Models. (2010) Semin Cell Dev Biol. Volume 21, Number 6 Pp. 655-662.
- ^ Direct utilization of mannose for mammalian glycoprotein biosynthesis. Oxford Journals, Life Sciences, Glycobiology, Volume 8, Number 3 Pp. 285-295.
- ^ Studies of Mannose Metabolism and Effects of Long-Term Mannose Ingestion in the Mouse (2001) Biochim Biophys Acta. Volume 1528, Number 2-3, Pp. 116-26.
- ^ Vlahopoulos S, Gritzapis AD, Perez SA, Cacoullos N, Papamichail M, Baxevanis CN. Mannose addition by yeast Pichia Pastoris on recombinant HER-2 protein inhibits recognition by the monoclonal antibody herceptin. Vaccine. 2009 Jul 23;27(34):4704-8. Epub 2009 Jun 9.PMID 19520203.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||