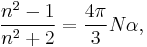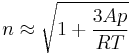Lorentz–Lorenz equation
The Lorentz–Lorenz equation, also known as the Clausius–Mossotti relation and Maxwell's formula, relates the refractive index of a substance to its polarizability.
The most general form of the Lorentz–Lorenz equation is
where 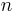 is the refractive index,
is the refractive index, 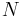 is the number of molecules per unit volume, and
is the number of molecules per unit volume, and 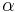 is the mean polarizability. This equation is only valid for certain crystal structures.[1][2]
is the mean polarizability. This equation is only valid for certain crystal structures.[1][2]
A more specialized form of the Lorentz–Lorenz equation gives the refractive index 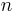 of a dilute gas as
of a dilute gas as
where 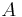 is the molar refractivity,
is the molar refractivity, 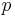 is the pressure of the gas,
is the pressure of the gas, 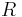 is the universal gas constant, and
is the universal gas constant, and 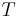 is the (absolute) temperature.
is the (absolute) temperature.
History
The Lorentz–Lorenz equation is named after the Danish mathematician and scientist Ludvig Lorenz, who published it in 1869, and the Dutch physicist Hendrik Lorentz, who discovered it independently in 1878.
See also
References
- Born, Max, and Wolf, Emil, Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light (7th ed.), section 2.3.3, Cambridge University Press (1999) ISBN 0-521-64222-1