Immunoglobulin G
Immunoglobulin G (IgG) are antibody molecules. Each IgG is composed of four peptide chains — two heavy chains γ and two light chains. Each IgG has two antigen binding sites. Other immunoglobulins may be described in terms of polymers with the IgG structure considered the monomer.
IgG constitutes 75% of serum immunoglobulins in humans.[1] IgG molecules are synthesized and secreted by plasma B cells.
Contents |
Functions
IgG antibodies are involved in predominantly the secondary immune response. The presence of specific IgG, in general, corresponds to maturation of the antibody response.[2] Human IgG Subclasses: IgG is the only isotype that can pass through the human placenta, thereby providing protection to the fetus in utero. Along with IgA secreted in the breast milk, residual IgG absorbed through the placenta provides the neonate with humoral immunity before its own immune system develops. Colostrum contains a high percentage of IgG, especially in bovine colostrum.
IgG can bind to many kinds of pathogens, for example viruses, bacteria, and fungi, and protects the body against them by agglutination and immobilization, complement activation (classical pathway), opsonization for phagocytosis, and neutralization of their toxins. It also plays an important role in Antibody-dependent cell-mediated cytotoxicity(ADCC) and Intracellular antibody-mediated proteolysis, in which it binds to TRIM21 (the receptor with greatest affinity to IgG in humans) in order to direct marked virions to the proteasome in the cytosol.[3]
IgG is also associated with Type II and Type III Hypersensitivity.
Structure
IgG antibodies are large molecules of about 150 kDa composed of four peptide chains. It contains two identical heavy chains of about 50 kDa and two identical light chains of about 25 kDa, thus a tetrameric quaternary structure. The two heavy chains are linked to each other and to a light chain each by disulfide bonds. The resulting tetramer has two identical halves, which together form the Y-like shape. Each end of the fork contains an identical antigen binding site. The Fc regions of IgGs bear a highly conserved N-glycosylation site. The N-glycans attached to this site are predominantly core-fucosylated diantennary structures of the complex type. In addition, small amounts of these N-glycans also bear bisecting GlcNAc and α-2,6-linked sialic acid residues.[4]
Low-frequency internal motion
Immunoglobulin G has a low-frequency wave number of 28 cm−1 in the Raman spectra.[5] This emission has been assigned to the breathing motion in the beta-barrel of nine beta-strands in its V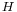 domain.[6] The dynamic mechanism of the "chelate effect" and "trigger effect" of IgG has been analyzed from the angle of low-frequency resonance among the 12 beta-barrels of an IgG molecule.[7]
domain.[6] The dynamic mechanism of the "chelate effect" and "trigger effect" of IgG has been analyzed from the angle of low-frequency resonance among the 12 beta-barrels of an IgG molecule.[7]
Subclasses
There are four IgG subclasses (IgG1, 2, 3, and 4) in humans, named in order of their abundance in serum (IgG1 being the most abundant).
| Name | Percent | Crosses placenta easily | Complement activator | Binds to Fc receptor on phagocytic cells |
| IgG1 | 66% | yes (1.47)† | second-highest | high affinity |
| IgG2 | 23% | no (0.8)† | third-highest | extremely low affinity |
| IgG3 | 7% | yes (1.17)† | highest | high affinity |
| IgG4 | 4% | yes (1.15)† | no | intermediate affinity |
| †: Quota cord/maternity concentrations blood. Based on data from a Japanese study on 228 mothers. [8] | ||||
Note: IgG affinity to Fc receptors on phagocytic cells is specific to individual species from which the antibody comes as well as the class. The structure of the hinge regions gives each of the 4 IgG classes its unique biological profile. Even though there is about 95% similarity between their Fc regions, the structure of the hinge regions is relatively different.
In a model of autoantibody mediated anemia using IgG isotype switch variants of an anti erythrocytes autoantibody, it was found that IgG2a was superior to IgG1 in activating complement. Moreover, it was found that the IgG2a isotype was able to interact very efficiently with FcgammaR. As a result, 20 times higher doses of IgG1 in relationship to IgG2a autoantibodies, were requried to induce autoantibody mediated pathology.
Azeredo et al. J of Exp Med. 2002. 195: 665.
See also
External links
- Janeway Immunobiology - The structure of a typical antibody (IgG)
- A booklet with everything you wanted to know about IgG subclasses
References
- ^ Junqueira, Luiz C.; Jose Carneiro (2003). Basic Histology. McGraw-Hill. ISBN 0838505902.
- ^ Meulenbroek, A.J.; Zeijlemaker, W.P. (1996).
- ^ Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC (2010). "Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21)". Proc. Natl. Acad. Sci. U.S.A. 107 (46): 19985–19990. doi:10.1073/pnas.1014074107. PMC 2993423. PMID 21045130. http://www.pnas.org/content/early/2010/11/01/1014074107.
- ^ Stadlmann J, Pabst M, Kolarich D, Kunert R, Altmann F. (2008). "Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides". Proteomics 8 (14): 2858–2871. doi:10.1002/pmic.200700968. PMID 18655055.
- ^ Painter PC, Mosher LE, Rhoads C (July 1982). "Low-frequency modes in the Raman spectra of proteins". Biopolymers 21 (7): 1469–72. doi:10.1002/bip.360210715. PMID 7115900.
- ^ Chou KC (August 1985). "Low-frequency motions in protein molecules. Beta-sheet and beta-barrel". Biophys. J. 48 (2): 289–97. doi:10.1016/S0006-3495(85)83782-6. PMC 1329320. PMID 4052563. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1329320.
- ^ Chou KC (February 1987). "The biological functions of low-frequency vibrations (phonons). VI. A possible dynamic mechanism of allosteric transition in antibody molecules". Biopolymers 26 (2): 285–95. doi:10.1002/bip.360260209. PMID 3828475.
- ^ Hashira S, Okitsu-Negishi S, Yoshino K (August 2000). "Placental transfer of IgG subclasses in a Japanese population". Pediatr Int 42 (4): 337–42. doi:10.1046/j.1442-200x.2000.01245.x. PMID 10986861.
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||