Hysteresivity
Hysteresivity derives from “hysteresis”, meaning “lag”. It is the tendency to react slowly to an outside force, or to not return completely to its original state. Whereas the area within a hysteresis loop represents energy dissipated to heat and is an extensive quantity with units of energy, the hysteresivity represents the fraction of the elastic energy that is lost to heat, and is an intensive property that is dimensionless.
Contents |
Overview
When a force deforms a material it generates elastic stresses and internal frictional stresses. Most often, frictional stress is described as being analogous to the stress that results from the flow of a viscous fluid, but in many engineering materials, in soft biological tissues, and in living cells, the concept that friction arises only from a viscous stress is now known to be erroneous.[1][2] For example, Bayliss and Robertson [3] and Hildebrandt [4] demonstrated that frictional stress in lung tissue is dependent upon the amount of lung expansion but not the rate of expansion, findings that are fundamentally incompatible with the notion of friction being caused by a viscous stress. If not by a viscous stress, how then does friction arise, and how is it properly described?
In many inert and living materials, the relationship between elastic and frictional stresses turns out to be very nearly invariant (something unaltered by a transformation). In lung tissues, for example, the frictional stress is almost invariably between 0.1 and 0.2 of the elastic stress, where this fraction is called the hysteresivity, h, or, equivalently, the structural damping coefficient.[2] It is a simple phenomenological fact, therefore, that for each unit of peak elastic strain energy that is stored during a cyclic deformation, 10 to 20 % of that elastic energy is taxed as friction and lost irreversibly to heat. This fixed relationship holds at the level of the whole lung [5] ,[6] isolated lung parenchymal tissue strips,[7] isolated smooth muscle strips,[2][8] and even isolated living cells.[9][10][11][12]
This close relationship between frictional and elastic stresses is called the structural damping law [1][2][4][13] or, sometimes, the constant phase model.[5] The structural damping law implies that frictional losses are coupled tightly to elastic stresses rather than to viscous stresses, but the precise molecular mechanical origin of this phenomenon remains unknown.[9][14]
In material science, the complex elastic modulus of a material, G*(f), at frequency of oscillatory deformation, f, is given by,
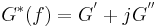
where:
- G*(f)= complex elastic modulus at frequency of oscillatory deformation, f
- G’ = the elastic modulus
- G” = the loss modulus
- j 2 = -1
This relationship can be rewritten as,
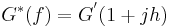
where:
- h = G”/G’.
In systems conforming to the structural damping law, the hysteresivity "h" is constant with or insensitive to changes in oscillatory frequency, and the loss modulus G” (= h G’) becomes a constant fraction of the elastic modulus.
See also
References
- ^ a b Crandall SH. The role of damping in vibration theory. J Sound and Vibration 11: 3-18, 1970.
- ^ a b c d Fredberg JJ and Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol 67: 2408-2419, 1989.
- ^ Bayliss L and Robertson G. The visco-elastic properties of the lungs. QJ Experimental Physiology (journal) 29, 1939.
- ^ a b Hildebrandt J. Comparison of mathematical models for cat lung and viscoelastic balloon derived by Laplace transform methods from pressure-volume data. Bull Math Biophys 31: 651-667, 1969.
- ^ a b Hantos Z, Daroczy B, Suki B, Nagy S, and Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168-178, 1992.
- ^ Jensen A, Atileh H, Suki B, Ingenito EP, and Lutchen KR. Airway caliber in healthy and asthmatic subjects: effects of bronchial challenge and deep inspirations. J Appl Physiol 91: 506-515; discussion 504-505, 2001.
- ^ Fredberg JJ, Bunk D, Ingenito E, and Shore SA. Tissue resistance and the contractile state of lung parenchyma. J Appl Physiol 74: 1387-1397, 1993.
- ^ Fredberg JJ, Jones KA, Nathan M, Raboudi S, Prakash YS, Shore SA, Butler JP, and Sieck GC. Friction in airway smooth muscle: mechanism, latch, and implications in asthma. J Appl Physiol 81: 2703-2712, 1996.
- ^ a b Bursac P, Lenormand G, Fabry B, Oliver M, Weitz DA, Viasnoff V, Butler JP, and Fredberg JJ. Cytoskeletal remodelling and slow dynamics in the living cell. Nat Mater 4: 557-571, 2005.
- ^ Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, and Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett 87: 148102, 2001.
- ^ Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Taback NA, Millet EJ, and Fredberg JJ. Time scale and other invariants of integrative mechanical behavior in living cells. Phys Rev E Stat Nonlin Soft Matter Phys 68: 041914, 2003.
- ^ Fabry B, Maksym GN, Shore SA, Moore PE, Panettieri RA, Jr., Butler JP, and Fredberg JJ. Time course and heterogeneity of contractile responses in cultured human airway smooth muscle cells. J Appl Physiol 91: 986-994., 2001.
- ^ Fung Y. Biomechanics: Mechanical Properties of Living Tissues. New York:: Springer-Verlag, 1988.
- ^ Hubmayr RD. Biology lessons from oscillatory cell mechanics. J Appl Physiol 89: 1617-1618, 2000.
Further reading
- Bayliss L and Robertson G. The visco-elastic properties of the lungs. QJ Experimental Physiology (journal) 29, 1939.
- Bursac P, Lenormand G, Fabry B, Oliver M, Weitz DA, Viasnoff V, Butler JP, and Fredberg JJ. Cytoskeletal remodelling and slow dynamics in the living cell. Nat Mater 4: 557-571, 2005.
- Crandall SH. The role of damping in vibration theory. J Sound and Vibration 11: 3-18, 1970.
- Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, and Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett 87: 148102, 2001.
- Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Taback NA, Millet EJ, and Fredberg JJ. Time scale and other invariants of integrative mechanical behavior in living cells. Phys Rev E Stat Nonlin Soft Matter Phys 68: 041914, 2003.
- Fabry B, Maksym GN, Shore SA, Moore PE, Panettieri RA, Jr., Butler JP, and Fredberg JJ. Time course and heterogeneity of contractile responses in cultured human airway smooth muscle cells. J Appl Physiol 91: 986-994., 2001.
- Fredberg JJ, Bunk D, Ingenito E, and Shore SA. Tissue resistance and the contractile state of lung parenchyma. J Appl Physiol 74: 1387-1397, 1993.
- Fredberg JJ, Jones KA, Nathan M, Raboudi S, Prakash YS, Shore SA, Butler JP, and Sieck GC. Friction in airway smooth muscle: mechanism, latch, and implications in asthma. J Appl Physiol 81: 2703-2712, 1996.
- Fredberg JJ and Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol 67: 2408-2419, 1989.
- Fung Y. Biomechanics: Mechanical Properties of Living Tissues. New York:: Springer-Verlag, 1988.
- Hantos Z, Daroczy B, Suki B, Nagy S, and Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168-178, 1992.
- Hildebrandt J. Comparison of mathematical models for cat lung and viscoelastic balloon derived by Laplace transform methods from pressure-volume data. Bull Math Biophys 31: 651-667, 1969.
- Hubmayr RD. Biology lessons from oscillatory cell mechanics. J Appl Physiol 89: 1617-1618, 2000.
- Jensen A, Atileh H, Suki B, Ingenito EP, and Lutchen KR. Airway caliber in healthy and asthmatic subjects: effects of bronchial challenge and deep inspirations. J Appl Physiol 91: 506-515; discussion 504-505, 2001.
- Kaczka DW, Ingenito EP, Suki B, Lutchen KR. Partitioning airway and lung tissue resistances in humans: effects of bronchoconstriction. J Appl Physiol 82: 1531-1541, 1997.