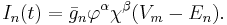Hodgkin–Huxley model
The Hodgkin–Huxley model is a mathematical model (a type of scientific model) that describes how action potentials in neurons are initiated and propagated. It is a set of nonlinear ordinary differential equations that approximates the electrical characteristics of excitable cells such as neurons and cardiac myocytes.
Alan Lloyd Hodgkin and Andrew Huxley described the model in 1952 to explain the ionic mechanisms underlying the initiation and propagation of action potentials in the squid giant axon.[1] They received the 1963 Nobel Prize in Physiology or Medicine for this work.
Contents |
Basic components
The components of a typical Hodgkin–Huxley model are shown in the figure. Each component of an excitable cell has a biophysical analog. The lipid bilayer is represented as a capacitance (Cm). Voltage-gated ion channels are represented by nonlinear electrical conductances (gn, where n is the specific ion channel), meaning that the conductance is voltage and time-dependent. This was later shown to be mediated by voltage-gated cation channel proteins, each of which has an open probability that is voltage-dependent. Leak channels are represented by linear conductances (gL). The electrochemical gradients driving the flow of ions are represented by batteries (En and EL), the values of which are determined from the Nernst potential of the ionic species of interest. Finally, ion pumps are represented by current sources (Ip).
The time derivative of the potential across the membrane (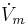 ) is proportional to the sum of the currents in the circuit. This is represented as follows:
) is proportional to the sum of the currents in the circuit. This is represented as follows:
where Ii denotes the individual ionic currents of the model.
Ionic current characterization
The current flowing through the ion channels is mathematically represented by the following equation:
where 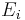 is the reversal potential of the i-th ion channel.
is the reversal potential of the i-th ion channel.
In voltage-gated ion channels, the channel conductance gi is a function of both time and voltage (gn(t, V) in the figure), while in leak channels gi is a constant (gL in the figure). The current generated by ion pumps is dependent on the ionic species specific to that pump. The following sections will describe these formulations in more detail.
Voltage-gated ion channels
Under the Hodgkin–Huxley formulation, conductances for voltage-gated channels (gn(t, V)) are expressed as:
where 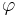 and
and 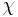 are gating variables for activation and inactivation, respectively, representing the fraction of the maximum conductance available at any given time and voltage.
are gating variables for activation and inactivation, respectively, representing the fraction of the maximum conductance available at any given time and voltage. 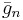 is the maximal value of the conductance.
is the maximal value of the conductance.  and
and 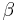 are constants and
are constants and 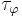 and
and 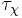 are the time constants for activation and inactivation, respectively.
are the time constants for activation and inactivation, respectively. 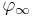 and
and 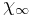 are the steady state values for activation and inactivation, respectively, and are usually represented by Boltzmann equations as functions of
are the steady state values for activation and inactivation, respectively, and are usually represented by Boltzmann equations as functions of 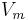 .
.
In order to characterize voltage-gated channels, the equations will be fit to voltage-clamp data. For a derivation of the Hodgkin–Huxley equations under voltage-clamp see.[2] Briefly, when the membrane potential is held at a constant value (i.e., voltage-clamp), for each value of the membrane potential the nonlinear gating equations reduce to linear differential equations of the form:
Thus, for every value of membrane potential, 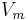 , the following equation can be fit to the current curve:
, the following equation can be fit to the current curve:
The Levenberg–Marquardt algorithm,[3][4] a modified Gauss–Newton algorithm, is often used to fit these equations to voltage-clamp data.
Leak channels
Leak channels account for the natural permeability of the membrane to ions and take the form of the equation for voltage-gated channels, where the conductance 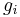 is a constant.
is a constant.
Pumps and exchangers
The membrane potential depends upon the maintenance of ionic concentration gradients across it. The maintenance of these concentration gradients requires active transport of ionic species. The sodium-potassium and sodium-calcium exchangers are the best known of these. Some of the basic properties of the Na/Ca exchanger have already been well-established: the stoichiometry of exchange is 3 Na+:1 Ca2+ and the exchanger is electrogenic and voltage-sensitive. The Na/K exchanger has also been described in detail.[5]
Improvements and alternative models
The Hodgkin–Huxley model is widely regarded as one of the great achievements of 20th-century biophysics. Nevertheless, modern Hodgkin–Huxley-type models have been extended in several important ways:
- Additional ion channel populations have been incorporated based on experimental data.
- Models often incorporate highly complex geometries of dendrites and axons, often based on microscopy data.
Several simplified neuronal models have also been developed (such as Fitzhugh-Nagumo model), facilitating efficient large-scale simulation of groups of neurons, as well as mathematical insight into dynamics of action potential generation.
See also
- Action potential
- Biological neural network
- Biological neuron model
- Fitzhugh-Nagumo model
- Memristor
- Soliton model
- Goldman equation
- GHK current equation
- Theta model
References
- ^ Hodgkin, A., and Huxley, A. (1952): A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117:500–544. PMID 12991237
- ^ Marquardt, D. (1963): An algorithm for the least-squares estimation of nonlinear parameters. SIAM J. Appl. Math. 11 (2):431–441.
- ^ Levenberg, K. (1944): A method for the solution of certain non-linear problems in least-squares. Q. Appl. Math. 2 (2):164–168.
- ^ Johnston, D., and Wu, S. (1997): Foundations of Cellular Neurophysiology, chapter 6. MIT Press, Cambridge, MA. ISBN 0-262-10053-3
- ^ Hille, B. (2001): Ionic Channels of Excitable Membranes (3rd ed.). Sinauer Associates, Inc., Sunderland, MA. ISBN 0-87893-321-2
External links
- Interactive Java applet of the HH model Parameters of the model can be changed as well as excitation parameters and phase space plottings of all the variables is possible.
- Direct link to Hodgkin-Huxley model and a Description in BioModels Database
- Direct link to Hodgkin-Huxley paper #1 via PubMedCentral
- Direct link to Hodgkin-Huxley paper #2 via PubMedCentral
- Direct link to Hodgkin-Huxley paper #3 via PubMedCentral
- Direct link to Hodgkin-Huxley paper #4 via PubMedCentral
- Direct link to Hodgkin-Huxley paper #5 via PubMedCentral
- Neural Impulses: The Action Potential In Action by Garrett Neske, The Wolfram Demonstrations Project
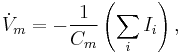
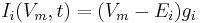
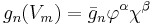
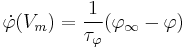
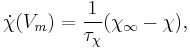
![\varphi(t) = \varphi_{0} - [ (\varphi_{0}-\varphi_{\infty})(1 - e^{-t/\tau_\varphi})]\,](/2012-wikipedia_en_all_nopic_01_2012/I/c72329aebff1bfb7d7fa76d9247c7f76.png)
![\chi(t) = \chi_{0} - [ (\chi_{0}-\chi_{\infty})(1 - e^{-t/\tau_\chi})].](/2012-wikipedia_en_all_nopic_01_2012/I/e3cf83154217ec4e64206722900d7491.png)