Ferrocyanide
Ferrocyanide is the name of the anion Fe(CN)64−. In aqueous solutions, this coordination complex is relatively unreactive. It is usually available as the salt potassium ferrocyanide, which has the formula K4Fe(CN)6.
Ferrocyanide was not named as a compound of iron and cyanide; rather cyanides as a chemical class were named because they were discovered in ferrocyanide. Ferrocyanide in turn was an iron radical found in the analysis of the intensely blue dye Prussian blue, and named in Latin to mean "blue substance with iron." The dye had been first accidentally made in the early 18th century from substances containing iron, carbon, and nitrogen, and the (then unknown) cyanide formed during the manufacture of the dye. The word "cyanide" used in the name is from kyanos, Greek for "(dark) blue." The ferrocyanide anion itself, when not complexed with iron cations, is colored a moderate yellow.
Contents |
Coordination chemistry
[Fe(CN)6]4− is a diamagnetic species, featuring low-spin iron(II) center in an octahedral ligand environment. Although many salts of cyanide are highly toxic, ferro- and ferricyanides are less toxic because they tend not to release free cyanide.[1] Its most important reaction is its oxidation to ferricyanide:
- [Fe(CN)6]4−
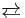 [Fe(CN)6]3− + e−
[Fe(CN)6]3− + e−
This conversion can be followed spectroscopically at 535 nm with an absorption coefficient of 21600 M−1 cm−1.
Treatment of ferrocyanide with ferric-containing salts gives the intensely coloured pigment Prussian Blue.[1]
Use in biochemical research
Ferrocyanide and its oxidized product ferricyanide, [Fe(CN)6]3−, are impermeable to the plasma membrane. For this reason ferrocyanide has been used as a probe of extracellular electron receptor in the study of redox reactions in cells. Ferricyanide is used thus any increase in ferrocyanide can be attributed to secretions of reductants or "Trans Plasma Membrane Electron Transport" (TPMET) activity.
Nomenclature
According to the recommendations of IUPAC, ferrocyanide should be called "hexacyanoferrate(II)."