Hemithioacetal
Hemithioacetal, also known as hemimercaptal, is an organic functional group with the general formula RCH(OR)SR.[1] They form in a spontaneous reaction between a thiol and an aldehyde. Since the formerly carbonyl carbon bears four different substituents, hemiacetals are chiral. Hemithioacetals are usually intermediates in the catalytic reactions and usually arise via acid or base catalysis. The hemithioacetal features vicinal hydroxyl and thioether functionalities. Hemithioacetals are usually unstable, degrading into thiol and aldehyde.
- RCHO + R’SH
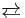 RCH(OH)(SR’)
RCH(OH)(SR’)
Hemithioacetals in nature
Glyoxalase I, which is part of the glyoxalase system present in the cytosol, catalyzes the conversion of α-oxoaldehyde (RCOCHO) and the thiol glutathione (GSH) to S-2-hydroxyacylglutathione derivatives [RCH(OH)CO-SG]. The catalytic mechanism involves an intermediate hemithioacetal adduct [RCOCH(OH)-SG]. The spontaneous reaction forms methylglyoxal-glutathione hemithioacetal and human glyoxalse I.[2]
A hemithioacetal is invoked in the mechanism of prenylcysteine lyase. In catalytic mechanism, S-farnesylcysteine is oxidized by flavin to a thiocarbenium ion. The thiocarbenium ion ([(RS)C(R’)(H)]+) hydrolyzes to form the hemithioacetal. After formation, the hemithioacetal breaks into hydrogen peroxide, farnesal, and cysteine.[3]
Isolable hemithioacetal
Hemithioacetals are ordinarily unstable because they readily dissociate into thiol and aldehyde. However, some isolable hemithioacetals have been reported. The few isolable hemithioacetals are all cyclic. The cyclic structure of the hemithioacetal disfavors dissociation. One example of the cyclic hemithioacetal is O-1-naphthylurethane. With 1-naphthyl isocyanate in the presence of triethylamine, tetrahydro-2-hydroxythiophen forms O-1-naphthylurethane, which retains cyclic form.[4] Another isolable hemithioacetal can be prepared by addition of thiol to methyl glyoxalate.[5] The stability of hemithioacetal is enhanced in the presence of acid, since any racemisation process (dissociation) is slowed.[6] The other approach to isolable hemithioacetals uses carbonyl groups that form stable hydrates. For example, a thiol (R’SH) reacts with hexafluoroacetone trihydrate. A stable hemithioacetal, which can be isolated, is obtained.[7]
Reference
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 0-471-72091-7, http://books.google.com/books?id=JDR-nZpojeEC&printsec=frontcover
- ^ Thornalley, P.J., Biochemical Society Transactions, Glyoxalase I - Structure, function and a critical role in the enzymatic defence against glycation, 2003, 31 (6), 1343-1348, ISSN: 03005127
- ^ Digits, J.A.; Pyun, H.-J.; Coates, R.M.; Casey, P.J. Journal of biological chemistry, Stereospecificity and kinetic mechanism of human prenylcysteine lyase, an unusual thioether oxidase, 2002, volume 277, 41086-41093, doi:10.1074/jbc.M208069200
- ^ Cox, J.M.; Owen, L.N., J. Chem. Soc. C, Cyclic hemithioacetals: Analogues of thiosugars with sulphur in the ring, 1967, 1130-1134. doi:10.1039/J39670001130
- ^ Milton, J; Brand, S; Jones, M.F; Rayner, C.M., Tetrahedron Letters, Enantioselective Enzymatic Synthesis of the Anti-Viral Agent Lamivudine, 1995, volume 36, 6961-6964, doi:10.1016/0040-4039(95)01380-Z
- ^ Barnett, R.E.; Jencks, W.P, J. Am. Chem. Soc, Diffusion-controlled and concerted catalysis in the decomposition of hemithioacetals, 1969, volume 91, 6758-6765, doi:10.1021/ja01052a038
- ^ Field, L.; Sweetman, B.J.; Bellas, M., Journal of Medicinal Chemistry, Biologically oriented organic sulfur chemistry. II. Formation of hemimercaptals or hemimercaptoles as a means of latentiating thiols, 1969, 12(4), 624-628. doi:10.1021/jm00304a014