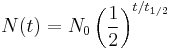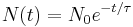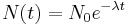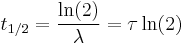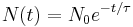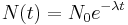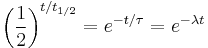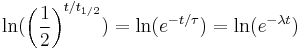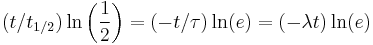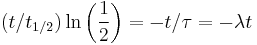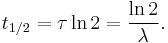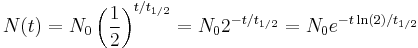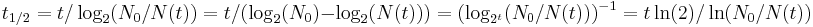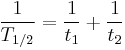Half-life
| Number of half-lives elapsed |
Fraction remaining |
Percentage remaining |
|
|---|---|---|---|
| 0 | 1/1 | 100 | |
| 1 | 1/2 | 50 | |
| 2 | 1/4 | 25 | |
| 3 | 1/8 | 12 | .5 |
| 4 | 1/16 | 6 | .25 |
| 5 | 1/32 | 3 | .125 |
| 6 | 1/64 | 1 | .563 |
| 7 | 1/128 | 0 | .781 |
| ... | ... | ... | |
| n | 1/(2n) | 100/(2n) | |
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms (radioactive decay), but it may apply to any quantity which follows a set-rate decay.
The original term, dating to 1907, was "half-life period", which was later shortened to "half-life" in the early 1950s.[1]
Half-lives are used to describe quantities undergoing exponential decay—for example, radioactive decay—where the half-life is constant over the whole life of the decay, and is a characteristic unit (a natural unit of scale) for the exponential decay equation. However, a half-life can also be defined for non-exponential decay processes, although in these cases the half-life varies throughout the decay process. For a general introduction and description of exponential decay, see the article exponential decay. For a general introduction and description of non-exponential decay, see the article rate law. Corresponding to sediments in environmental processes, if the half-life is greater than the residence time, then the radioactive nuclide will have enough time to significantly alter the concentration. The converse of half-life is doubling time.
The table on the right shows the reduction of a quantity in terms of the number of half-lives elapsed.
Contents |
Probabilistic nature of half-life
A half-life describes the decay of discrete entities, such as radioactive atoms. In that case, it does not work to use the definition "half-life is the time required for exactly half of the entities to decay". For example, if there is just one radioactive atom with a half-life of 1 second, there will not be "half of an atom" left after 1 second. There will be either zero atoms left or one atom left, depending on whether or not the atom happens to decay.
Instead, the half-life is defined in terms of probability. It is the time when the expected value of the number of entities that have decayed is equal to half the original number. For example, one can start with a single radioactive atom, wait its half-life, and measure whether or not it decays in that period of time. Perhaps it will and perhaps it will not. But if this experiment is repeated again and again, it will be seen that - on average - it decays within the half-life 50% of the time.
In some experiments (such as the synthesis of a superheavy element), there is in fact only one radioactive atom produced at a time, with its lifetime individually measured. In this case, statistical analysis is required to infer the half-life. In other cases, a very large number of identical radioactive atoms decay in the time-range measured. In this case, the law of large numbers ensures that the number of atoms that actually decay is essentially equal to the number of atoms that are expected to decay. In other words, with a large enough number of decaying atoms, the probabilistic aspects of the process can be ignored.
There are various simple exercises that demonstrate probabilistic decay, for example involving flipping coins or running a computer program.[2][3][4] For example, the image on the right is a simulation of many identical atoms undergoing radioactive decay. Note that after one half-life there are not exactly one-half of the atoms remaining, only approximately, due to random variation in the process. However, with more atoms (right boxes), the overall decay is smoother and less random than with fewer atoms (left boxes), in accordance with the law of large numbers.
Formulas for half-life in exponential decay
An exponential decay process can be described by any of the following three equivalent formulas:
where
-
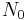 is the initial quantity of the substance that will decay (this quantity may be measured in grams, moles, number of atoms, etc.),
is the initial quantity of the substance that will decay (this quantity may be measured in grams, moles, number of atoms, etc.),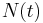 is the quantity that still remains and has not yet decayed after a time t,
is the quantity that still remains and has not yet decayed after a time t,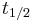 is the half-life of the decaying quantity,
is the half-life of the decaying quantity,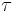 is a positive number called the mean lifetime of the decaying quantity,
is a positive number called the mean lifetime of the decaying quantity,- λ is a positive number called the decay constant of the decaying quantity.
The three parameters 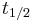 ,
, 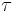 , and λ are all directly related in the following way:
, and λ are all directly related in the following way:
where ln(2) is the natural logarithm of 2 (approximately 0.693).
-
Click "show" to see a detailed derivation of the relationship between half-life, decay time, and decay constant. Start with the three equations We want to find a relationship between
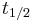 ,
, 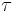 , and λ, such that these three equations describe exactly the same exponential decay process. Comparing the equations, we find the following condition:
, and λ, such that these three equations describe exactly the same exponential decay process. Comparing the equations, we find the following condition:Next, we'll take the natural logarithm of each of these quantities.
Using the properties of logarithms, this simplifies to the following:
Since the natural logarithm of e is 1, we get:
Canceling the factor of t and plugging in
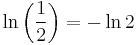 , the eventual result is:
, the eventual result is:
By plugging in and manipulating these relationships, we get all of the following equivalent descriptions of exponential decay, in terms of the half-life:
Regardless of how it's written, we can plug into the formula to get
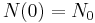 as expected (this is the definition of "initial quantity")
as expected (this is the definition of "initial quantity")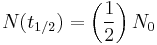 as expected (this is the definition of half-life)
as expected (this is the definition of half-life)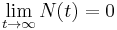 , i.e. amount approaches zero as t approaches infinity as expected (the longer we wait, the less remains).
, i.e. amount approaches zero as t approaches infinity as expected (the longer we wait, the less remains).
Decay by two or more processes
Some quantities decay by two exponential-decay processes simultaneously. In this case, the actual half-life T1/2 can be related to the half-lives t1 and t2 that the quantity would have if each of the decay processes acted in isolation:
For three or more processes, the analogous formula is:
For a proof of these formulas, see Decay by two or more processes.
Examples
-
Main article: Exponential decay--Applications and examples
There is a half-life describing any exponential-decay process. For example:
- The current flowing through an RC circuit or RL circuit decays with a half-life of
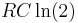 or
or 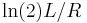 , respectively.
, respectively. - In a first-order chemical reaction, the half-life of the reactant is
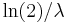 , where λ is the reaction rate constant.
, where λ is the reaction rate constant. - In radioactive decay, the half-life is the length of time after which there is a 50% chance that an atom will have undergone nuclear decay. It varies depending on the atom type and isotope, and is usually determined experimentally.
Half-life in non-exponential decay
The decay of many physical quantities is not exponential—for example, the evaporation of water from a puddle, or (often) the chemical reaction of a molecule. In such cases, the half-life is defined the same way as before: as the time elapsed before half of the original quantity has decayed. However, unlike in an exponential decay, the half-life depends on the initial quantity, and the prospective half-life will change over time as the quantity decays.
As an example, the radioactive decay of carbon-14 is exponential with a half-life of 5730 years. A quantity of carbon-14 will decay to half of its original amount after 5730 years, regardless of how big or small the original quantity was. After another 5730 years, one-quarter of the original will remain. On the other hand, the time it will take a puddle to half-evaporate depends on how deep the puddle is. Perhaps a puddle of a certain size will evaporate down to half its original volume in one day. But on the second day, there is no reason to expect that one-quarter of the puddle will remain; in fact, it will probably be much less than that. This is an example where the half-life reduces as time goes on. (In other non-exponential decays, it can increase instead.)
The decay of a mixture of two or more materials which each decay exponentially, but with different half-lives, is not exponential. Mathematically, the sum of two exponential functions is not a single exponential function. A common example of such a situation is the waste of nuclear power stations, which is a mix of substances with vastly different half-lives. Consider a sample containing a rapidly decaying element A, with a half-life of 1 second, and a slowly decaying element B, with a half-life of one year. After a few seconds, almost all atoms of the element A have decayed after repeated halving of the initial total number of atoms; but very few of the atoms of element B will have decayed yet as only a tiny fraction of a half-life has elapsed. Thus, the mixture taken as a whole does not decay by halves.
Half-life in biology and pharmacology
A biological half-life or elimination half-life is the time it takes for a substance (drug, radioactive nuclide, or other) to lose half of its pharmacologic, physiologic, or radiological activity. In a medical context, half-life may also describe the time it takes for the blood plasma concentration of a substance to halve ("plasma half-life") its steady-state. The relationship between the biological and plasma half-lives of a substance can be complex, due to factors including accumulation in tissues, active metabolites, and receptor interactions.[5]
While a radioactive isotope decays perfectly according to first order kinetics where the rate constant is fixed, the elimination of a substance from a living organism follows more complex kinetics.
For example, the biological half-life of water in a human is about 7 to 14 days, though this can be altered by behavior. The biological half-life of caesium in humans is between one and four months. This can be shortened by feeding the person Prussian blue, which acts as a solid ion exchanger which absorbs the caesium while releasing potassium ions.
See also
References
- ^ John Ayto, "20th Century Words" (1989), Cambridge University Press.
- ^ MADSCI.org
- ^ Exploratorium.edu
- ^ Astro.GLU.edu
- ^ Lin VW; Cardenas DD (2003). Spinal cord medicine. Demos Medical Publishing, LLC. p. 251. ISBN 1888799617. http://books.google.co.uk/books?id=3anl3G4No_oC&pg=PA251&lpg=PA251.
External links
- Nucleonica.net, Nuclear Science Portal
- Nucleonica.net, wiki: Decay Engine
- Bucknell.edu, System Dynamics - Time Constants
- Subotex.com, Half-Life elimination of drugs in blood plasma - Simple Charting Tool
|
||||||||||||||||||||||