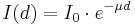Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays (especially in astronomy, by analogy with X-rays) and denoted as γ, is electromagnetic radiation of high frequency (very short wavelength). Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei (gamma decay). Important natural sources are also high-energy sub-atomic particle interactions resulting from cosmic rays. Such high-energy reactions are also the common artificial source of gamma rays. Other man-made mechanisms include electron-positron annihilation, neutral pion decay, fusion, and induced fission. Some rare natural sources are lightning strikes and terrestrial gamma-ray flashes, which produce high energy particles from natural high-energy voltages. Gamma rays are also produced by astronomical processes in which very high-energy electrons are produced. Such electrons produce secondary gamma rays by the mechanisms of bremsstrahlung, inverse Compton scattering and synchrotron radiation. Gamma rays are ionizing radiation and are thus biologically hazardous.
A classical gamma ray source, and the first to be discovered historically, is a type of radioactive decay called gamma decay. In this type of decay, an excited nucleus emits a gamma ray almost immediately on formation, although isomeric transition can produce inhibited gamma decay with a measurable and much longer half-life. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium.[1][2] Villard's radiation was named "gamma rays" by Ernest Rutherford in 1903.[3]
Gamma rays typically have frequencies above 10 exahertz (or >1019 Hz), and therefore have energies above 100 keV and wavelength less than 10 picometers, less than the diameter of an atom. However, this is not a hard and fast definition but rather only a rule-of-thumb description for natural processes. Gamma rays from radioactive decay commonly have energies of a few hundred keV, and almost always less than 10 MeV. On the other side of the decay energy range, there is effectively no lower limit to gamma energy derived from radioactive decay. By contrast, energies from astronomical sources can be much higher, ranging over 10 TeV (this is far too large to result from radioactive decay).[4]
| Nuclear physics |
|---|
| Radioactive decay Nuclear fission Nuclear fusion |
|
Classical decays
|
|
Advanced decays
|
|
Emission processes
|
|
Capturing
|
|
High energy processes
|
The distinction between X-rays and gamma rays has changed in recent decades. Originally, the electromagnetic radiation emitted by X-ray tubes almost invariably had a longer wavelength than the radiation emitted by radioactive nuclei (gamma rays).[5] Older literature distinguished between X- and gamma radiation on the basis of wavelength, with radiation shorter than some arbitrary wavelength, such as 10−11 m, defined as gamma rays.[6] However, with artificial sources now able to duplicate any electromagnetic radiation that originates in the nucleus, as well as far higher energies, the wavelengths characteristic of radioactive gamma ray sources vs. other types, now completely overlaps. Thus, gamma rays are now usually distinguished by their origin: X-rays are emitted by definition by electrons outside the nucleus, while gamma rays are emitted by the nucleus.[5][7][8][9] Exceptions to this convention occur in astronomy, where high energy processes known to involve other than radioactive decay are still named as sources of gamma radiation. A notable example is extremely powerful bursts of high-energy radiation normally referred to as long duration gamma-ray bursts, which produce gamma rays by a mechanism not compatible with radioactive decay. These bursts of gamma rays, thought to be due to collapse of stars called hypernovas, are the most powerful single events so far discovered in the cosmos.
Contents |
Naming conventions and overlap in terminology
In the past, the distinction between X-rays and gamma rays was based on energy (or equivalently frequency or wavelength), with gamma rays being considered a higher-energy version of X-rays. However, modern high-energy (megavoltage) X-rays produced by linear accelerators ("linacs") for megavoltage treatment in cancer radiotherapy, usually have higher energy (typically 4 to 25 MeV) than do most classical gamma rays produced by radioactive gamma decay. Conversely, one of the most common gamma ray emitting isotopes used in diagnostic nuclear medicine, technetium-99m, produces gamma radiation of about the same energy (140 keV) as produced by a diagnostic X-ray machine, and significantly lower energy than therapeutic photons from linacs.
Because of this broad overlap in energy ranges, the two types of electromagnetic radiation are now usually defined by their origin: X-rays are emitted by electrons (either in orbitals outside of the nucleus, or while being accelerated to produce Bremsstrahlung-type radiation), while gamma rays are emitted by the nucleus or from other particle decays or annihilation events. There is no lower limit to the energy of photons produced by nuclear reactions, and thus ultraviolet and even lower energy photons produced by these processes would also be defined as "gamma rays". [11] This rule that electromagnetic radiation that is known to be of atomic nuclear origin (i.e., from gamma radioactive decay) is always referred to as "gamma rays," never X-rays, is the only naming-convention that is still universally respected. However, the reverse naming-convention that all gamma rays are of nuclear origin, is frequently violated.
In astronomy, higher energy gamma and X-rays are defined by energy, since the processes which produce them may be uncertain and photon energy, not origin, determines the required astronomical detectors needed. [12] Occasionally, high energy photons in nature which are known not to be produced by nuclear decay, are nevertheless referred to as gamma radiation. An example is "gamma rays" from lightning discharges at 10 to 20 MeV, which are known to be produced by the Bremsstrahlung mechanism.
Another example is gamma ray bursts, which are named historically, and now known to be produced from processes too powerful to involve simple collections of atoms undergoing radioactive decay. This has led to the realization that many gamma rays produced in astronomical processes are produced not in radioactive decay or particle annihilation, but rather in much the same manner as the production of X-rays, but simply using electrons with higher energies. However, a few gamma rays known to be explicitly from nuclear origin (by their spectra and half-life) are known in astronomy, with a classic example being that of supernova SN 1987A emitting an "afterglow" of gamma-ray photons from the decay of newly-made radioactive cobalt-56 ejected into space in a cloud, by the explosion. Astronomical literature tends to write "gamma-ray" with a hyphen, by analogy to X-rays, rather than in a way analogous to alpha rays and beta rays. This notation tends to subtley stress the non-nuclear source of most astronomical gamma rays.
Units of measure and exposure
The measure of gamma rays' ionizing ability is called the exposure:
- The coulomb per kilogram (C/kg) is the SI unit of ionizing radiation exposure, and is the amount of radiation required to create 1 coulomb of charge of each polarity in 1 kilogram of matter.
- The röntgen (R) is an obsolete traditional unit of exposure, which represented the amount of radiation required to create 1 esu of charge of each polarity in 1 cubic centimeter of dry air. 1 röntgen = 2.58×10−4 C/kg
However, the effect of gamma and other ionizing radiation on living tissue is more closely related to the amount of energy deposited rather than the charge. This is called the absorbed dose:
- The gray (Gy), which has units of (J/kg), is the SI unit of absorbed dose, and is the amount of radiation required to deposit 1 joule of energy in 1 kilogram of any kind of matter.
- The rad is the (obsolete) corresponding traditional unit, equal to 0.01 J deposited per kg. 100 rad = 1 Gy.
The equivalent dose is the measure of the biological effect of radiation on human tissue. For gamma rays it is equal to the absorbed dose.
- The sievert (Sv) is the SI unit of equivalent dose, which for gamma rays is numerically equal to the gray (Gy).
- The rem is the traditional unit of equivalent dose. For gamma rays it is equal to the rad or 0.01 J of energy deposited per kg. 1 Sv = 100 rem.
Properties
Shielding
Shielding from gamma rays requires large amounts of mass, in contrast to alpha particles which can be blocked by paper or skin, and beta particles which can be shielded by foil. They are better absorbed by materials with high atomic numbers and high density, although neither effect is important compared to the total mass per area in the path of the gamma ray. For this reason, a lead shield is only modestly better (20–30% better) as a gamma shield, than an equal mass of another shielding material such as aluminium, concrete, water or soil; lead's major advantage is not in lower weight, but rather its compactness due to higher density. Protective clothing, goggles and respirators can protect from internal contact with or ingestion of alpha or beta particles, but provide no protection from gamma radiation.
The higher the energy of the gamma rays, the thicker the shielding required. Materials for shielding gamma rays are typically measured by the thickness required to reduce the intensity of the gamma rays by one half (the half value layer or HVL). For example gamma rays that require 1 cm (0.4″) of lead to reduce their intensity by 50% will also have their intensity reduced in half by 4.1 cm of granite rock, 6 cm (2½″) of concrete, or 9 cm (3½″) of packed soil. However, the mass of this much concrete or soil is only 20–30% larger than that of lead with the same absorption capability. Depleted uranium is used for shielding in portable gamma ray sources, but again the savings in weight over lead is modest, and the main effect is to reduce shielding bulk. In a nuclear power plant, shielding can be provided by steel and concrete in the pressure vessel and containment, while water also provides a shielding material for fuel rods in storage or transport into the reactor core. A loss of water or removal of a "hot" spent fuel assembly into the air would result in much higher radiation levels than under water.
Matter interaction
When a gamma ray passes through matter, the probability for absorption in a thin layer is proportional to the thickness of that layer. Thus, if a beam of gamma rays passes through a thick slab of material, the scattering from the sides reduces intensity that reaches each element, so that the total absorption to an exponential decrease of intensity with thickness:
where μ = nσ is the absorption coefficient, measured in cm−1, n the number of atoms per cm3 in the material, σ the absorption cross section in cm2 and d the thickness of material in cm.
In passing through matter, gamma radiation ionizes via three main processes: the photoelectric effect, Compton scattering, and pair production.
- Photoelectric effect: This describes the case in which a gamma photon interacts with and transfers its energy to an atomic electron, ejecting that electron from the atom. The kinetic energy of the resulting photoelectron is equal to the energy of the incident gamma photon minus the binding energy of the electron. The photoelectric effect is the dominant energy transfer mechanism for X-ray and gamma ray photons with energies below 50 keV (thousand electron volts), but it is much less important at higher energies.
- Compton scattering: This is an interaction in which an incident gamma photon loses enough energy to an atomic electron to cause its ejection, with the remainder of the original photon's energy being emitted as a new, lower energy gamma photon with an emission direction different from that of the incident gamma photon. The probability of Compton scatter decreases with increasing photon energy. Compton scattering is thought to be the principal absorption mechanism for gamma rays in the intermediate energy range 100 keV to 10 MeV. Compton scattering is relatively independent of the atomic number of the absorbing material, which is why very dense metals like lead are only modestly better shields, on a per weight basis, than are less dense materials.
- Pair production: This becomes possible with gamma energies exceeding 1.02 MeV, and becomes important as an absorption mechanism at energies over about 5 MeV (see illustration at right, for lead). By interaction with the electric field of a nucleus, the energy of the incident photon is converted into the mass of an electron-positron pair. Any gamma energy in excess of the equivalent rest mass of the two particles (1.02 MeV) appears as the kinetic energy of the pair and the recoil nucleus. At the end of the positron's range, it combines with a free electron. The entire mass of these two particles is then converted into two gamma photons of at least 0.51 MeV energy each (or higher according to the kinetic energy of the annihilated particles).
The secondary electrons (and/or positrons) produced in any of these three processes frequently have enough energy to produce much ionization themselves.
Light interaction
High-energy (from 80 to 500 GeV) gamma rays arriving from far far-distant quasars are used to estimate the extragalactic background light in the universe: The highest-energy rays interact more readily with the background light photons and thus their density may be estimated by analyzing the incoming gamma ray spectrums.[13]
Gamma ray production
Gamma rays can be produced by a wide range of phenomena.
Radioactive decay (gamma decay)
Gamma rays from radioactive gamma decay are produced alongside other forms of radiation such as alpha or beta, and are produced after the other types of decay occur. The mechanism is that when a nucleus emits an α or β particle, the daughter nucleus is usually left in an excited state. It can then move to a lower energy state by emitting a gamma ray, in much the same way that an atomic electron can jump to a lower energy state by emitting infrared, visible, or ultraviolet light. Emission of a gamma ray from an excited nuclear state typically requires only 10−12 seconds, and is thus nearly instantaneous, following types of radioactive decay that produce other radioactive particles. Gamma decay from excited states may also happen rapidly following nuclear reactions such as neutron capture, nuclear fission, or nuclear fusion.
In certain cases, the excited nuclear state following the emission of a beta particle may be more stable than average, and is termed a metastable excited state, if its decay is 100 to 1000 times longer than the average 10−12 seconds. Such nuclei have half-lives that are easily measurable, and are termed nuclear isomers. Some nuclear isomers are able to stay in their excited state for minutes, hours, days, or occasionally far longer, before emitting a gamma ray. Isomeric transition is the name given to a gamma decay from such a state. The process of isomeric transition is therefore similar to any gamma emission, but differs in that it involves metastable excited states of the nuclei.
An emitted gamma ray from any type of excited state may transfer its energy directly to one of the most tightly bound electrons causing it to be ejected from the atom, a process termed the photoelectric effect (it should not be confused with the internal conversion process, in which no real gamma ray photon is produced as an intermediate particle).
Gamma rays, X-rays, visible light, and radio waves are all forms of electromagnetic radiation. The only difference is the frequency and hence the energy of the photons. Gamma rays are generally the most energetic of these, although broad overlap with X-ray energies occurs. An example of gamma ray production follows:
First 60
Co decays to excited 60
Ni by beta decay. Then the 60
Ni drops down to the ground state (see nuclear shell model) by emitting two gamma rays in succession (1.17 MeV then 1.33 MeV):
Another example is the alpha decay of 241
Am to form 237
Np; this alpha decay is accompanied by gamma emission. In some cases, the gamma emission spectrum for a nucleus (daughter nucleus) is quite simple, (e.g. 60
Co/60
Ni) while in other cases, such as with (241
Am/237
Np and 192
Ir/192
Pt), the gamma emission spectrum is complex, revealing that a series of nuclear energy levels can exist. The fact that an alpha spectrum can have a series of different peaks with different energies reinforces the idea that several nuclear energy levels are possible.
Because a beta decay is accompanied by the emission of a neutrino which also carries energy away, the beta spectrum does not have sharp lines, but instead is a broad peak. Hence from beta decay alone it is not possible to probe the different energy levels found in the nucleus.
In optical spectroscopy, it is well known that an entity which emits light can also absorb light at the same wavelength (photon energy). For instance, a sodium flame can emit yellow light as well as absorb the yellow light from a sodium vapor lamp. In the case of gamma rays, this can be seen in Mössbauer spectroscopy. Here, a correction for the Doppler shift due to recoil of the nucleus usually is not required, since the emitting and absorbing atoms are locked into a crystal, which absorbs their momentum (see Mössbauer effect. In this way, the exact conditions for gamma ray absorption through resonance can be attained.
This is similar to the Franck Condon effects seen in optical spectroscopy.
Gamma rays from sources other than radioactive decay
Gamma radiation, like X-radiation, can be produced by a variety of phenomena. For example, when high-energy gamma rays, electrons, or protons bombard materials, the excited atoms within emit characteristic "secondary" (or fluorescent) gamma rays, which are products of temporary creation of excited nuclear states in the bombarded atoms (such transitions form a topic in nuclear spectroscopy). Such gamma rays are produced by the nucleus, but not as a result of nuclear excitement from radioactive decay.
Energy in the gamma radiation range, often explicitly called gamma-radiation when it comes from astrophysical sources, is also produced by sub-atomic particle and particle-photon interactions. These include electron-positron annihilation, neutral pion decay, bremsstrahlung, inverse Compton scattering and synchrotron radiation. In a terrestrial gamma-ray flash a brief pulse of gamma radiation occurring high in the atmosphere of Earth, gamma rays are thought to be produced by high intensity static electric fields accelerating electrons, which then produce gamma rays by bremsstrahlung interactions with atoms in the air they collide with.
High energy gamma rays in astronomy include a gamma ray background produced when cosmic rays (either high speed electrons or protons) interact with ordinary matter, producing both pair-production gamma rays at 511 keV, or bremsstrahlung at energies of tens of MeV or more, when cosmic ray electrons interact with nuclei of sufficiently high atomic number (see gamma ray image of the Moon at the beginning of this article, for illustration).
- Pulsars and magnetars. The gamma ray sky (see illustration at right) is dominated by the more common and longer-term production of gamma rays in beams that emanate from pulsars within the Milky Way. Sources from the rest of the sky are mostly quasars. Pulsars are thought to be neutron stars with magnetic fields that produce focused beams of radiation, and are far less energetic, more common, and much nearer (typically seen only in our own galaxy) than are quasars (or the rarer sources of gamma ray bursts discussed below). In a pulsar, which produces gamma rays for much longer than a burst, the relatively long-lived magnetic field of the pulsar produces the focused beams of relativistic charged particles, which produce gamma rays in interaction with matter, when these charged particles strike gas or dust in the nearby medium, and are deflected or stopped. This is a similar mechanism to the production of high energy photons megavoltage radiation therapy machines (see bremsstrahlung). The "inverse Compton effect," in which charged particles (usually electrons) scatter from low-energy photons to convert them to higher energy photons (the gamma rays) is another possible mechanism of gamma ray production from relativistic charged particle beams. Neutron stars with a very high magnetic field (magnetars) are thought to produce astronomical soft gamma repeaters, which are another relatively long-lived neutron star-powered source of gamma radiation.
- Quasars and active galaxies. More powerful gamma rays from much farther quasars and other active galaxies probably have a roughly similar linear particle accelerator-like method of production, with high energy electrons produced by the quasar, followed again by inverse Compton scattering, synchrotron radiation, or bremsstrahlung, to produce gamma rays. However, quasar gamma rays are produced from a distance much further away, in distant galaxies. As the black hole at the center of such galaxies intermittantly destroys stars and focuses charged particles derived from them into beams, these beams interact with gas, dust, and lower energy photons to produce X-ray and gamma ray radiation. These sources are known to fluctuate with durations of a few weeks, indicating their relatively small size (less than a few light-weeks across). The particle beams emerge from the rotatational poles of the supermassive black hole at a galactic center, which is thought to form the power source of the quasar. Such sources of gamma and X-rays are the most commonly-visible high intensity sources outside our own galaxy, since they shine not as bursts (see illustration), but instead relatively continuously when viewed with gamma ray telescopes. The power of a typical quasar is about 1040 watts, of which only a small fraction is emitted as gamma radiation, and much of the rest is emitted as electromagnetic waves at all frequencies, including radio waves.
- Gamma-ray bursts. The most intense sources of gamma rays known, are also the most intense sources of any type of electromagnetic radiation presently known. They are rare compared with the sources discussed above. These intense sources are the "long duration burst" sources of gamma rays in astronomy ("long" in this context, meaning a few tens of seconds). By contrast, "short" gamma ray bursts, which are not associated with supernovae, are thought to produce gamma rays during the collision of pairs of neutron stars, or a neutron star and black hole after they spiral toward each other by emission of gravitational waves; such bursts last two seconds or less, and are of far lower energy than the "long" bursts (they are often seen only in our own galaxy for this reason). [14]
The so-called long duration gamma ray bursts produce events in which energies of ~ 1044 joules (as much energy as our Sun will produce in its entire life-time) but over a period of only 20 to 40 seconds, accompanied by high-efficiency conversion to gamma rays (on the order of 50% total energy conversion). The leading hypotheses for the mechanism of production of these highest-known intensity beams of radiation, are inverse Compton scattering and synchrotron radiation production of gamma rays from high-energy charged particles. These processes occur as relativistic charged particles leaving the region near the event horizon of the newly-formed black hole during the supernova explosion, and focused for a few tens of seconds into a relativistic beam by the magnetic field of the exploding hypernova. The fusion explosion of the hypernova drives the energetics of the process. If the beam happens to be narrowly directed in the direction of the Earth, it shines with high gamma ray power even at distances of up to 10 billion light years—close to the edge of the visible universe.
Health effects
All ionizing radiation causes similar damage at a cellular level, but because rays of alpha particles and beta particles are relatively non-penetrating, external exposure to them causes only localized damage, e.g. radiation burns to the skin. Gamma rays and neutrons are more penetrating, causing diffuse damage throughout the body (e.g. radiation sickness), increasing incidence of cancer rather than burns. External radiation exposure should also be distinguished from internal exposure, due to ingested or inhaled radioactive substances, which, depending on the substance's chemical nature, can produce both diffuse and localized internal damage. The most biological damaging forms of gamma radiation occur in the gamma ray window, between 3 and 10 MeV, with higher energy gamma rays being less harmful because the body is relatively transparent to them. See cobalt-60.
Uses
Gamma rays travel to Earth across vast distances of the universe, only to be absorbed by Earth's atmosphere. Different wavelengths of light penetrate Earth's atmosphere to different depths. Instruments aboard high-altitude balloons and such satellites as the Compton Observatory provide our only view of the gamma spectrum sky.
Gamma-induced molecular changes can also be used to alter the properties of semi-precious stones, and is often used to change white topaz into blue topaz.
Non-contact industrial sensors used in the Refining, Mining, Chemical, Food, Soaps and Detergents, and Pulp and Paper industries, in applications measuring levels, density, and thicknesses commonly use sources of gamma. Typically these use Co-60 or Cs-137 isotopes as the radiation source.
In the US, gamma ray detectors are beginning to be used as part of the Container Security Initiative (CSI). These US$5 million machines are advertised to scan 30 containers per hour. The objective of this technique is to screen merchant ship containers before they enter US ports.
Gamma radiation is often used to kill living organisms, in a process called irradiation. Applications of this include sterilizing medical equipment (as an alternative to autoclaves or chemical means), removing decay-causing bacteria from many foods or preventing fruit and vegetables from sprouting to maintain freshness and flavor.
Despite their cancer-causing properties, gamma rays are also used to treat some types of cancer, since the rays kill cancer cells also. In the procedure called gamma-knife surgery, multiple concentrated beams of gamma rays are directed on the growth in order to kill the cancerous cells. The beams are aimed from different angles to concentrate the radiation on the growth while minimizing damage to surrounding tissues.
Gamma rays are also used for diagnostic purposes in nuclear medicine in imaging techniques. A number of different gamma-emitting radioisotopes are used. For example, in a PET scan a radiolabled sugar called fludeoxyglucose emits positrons that are converted to pairs of gamma rays that localize cancer (which often takes up more sugar than other surrounding tissues). The most common gamma emitter used in medical applications is the nuclear isomer technetium-99m which emits gamma rays in the same energy range as diagnostic X-rays. When this radionuclide tracer is administered to a patient, a gamma camera can be used to form an image of the radioisotope's distribution by detecting the gamma radiation emitted (see also SPECT). Depending on what molecule has been labeled with the tracer, such techniques can be employed to diagnose a wide range of conditions (for example, the spread of cancer to the bones in a bone scan).
Body response
When gamma radiation breaks DNA molecules, a cell may be able to repair the damaged genetic material, within limits. However, a study of Rothkamm and Lobrich has shown that this repair process works well after high-dose exposure but is much slower in the case of a low-dose exposure.[15]
Risk assessment
The natural outdoor exposure in Great Britain ranges from 2 to 4 nSv/h (nanosieverts per hour).[16] Natural exposure to gamma rays is about 1 to 2 mSv per year, and the average total amount of radiation received in one year per inhabitant in the USA is 3.6 mSv.[17] There is a small increase in the dose, due to naturally occurring gamma radiation, around small particles of high atomic number materials in the human body caused by the photoelectric effect.[18]
By comparison, the radiation dose from chest radiography (about 0.06 mSv) is a fraction of the annual naturally occurring background radiation dose,.[19] A chest CT delivers 5 to 8 mSv. A whole-body PET/CT scan can deliver 14 to 32 mSv depending on the protocol.[20] The dose from fluoroscopy of the stomach is much higher, approximately 50 mSv (14 times the annual yearly background).
An acute full-body equivalent single exposure dose of 1 Sv (1000 mSv) causes slight blood changes, but 2.0–3.5 Sv (2.0–3.5 Gy) causes very severe syndrome of nausea, hair loss, and hemorrhaging, and will cause death in a sizable number of cases—-about 10% to 35% without medical treatment. A dose of 5 Sv[21] (5 Gy) is considered approximately the LD50 (lethal dose for 50% of exposed population) for an acute exposure to radiation even with standard medical treatment. A dose higher than 5 Sv (5 Gy) brings an increasing chance of death above 50%. Above 7.5–10 Sv (7.5–10 Gy) to the entire body, even extraordinary treatment, such as bone-marrow transplants, will not prevent the death of the individual exposed (see Radiation poisoning).. (Doses much larger than this may, however, be delivered to selected parts of the body in the course of radiation therapy.)
For low dose exposure, for example among nuclear workers, who receive an average yearly radiation dose of 19 mSv, the risk of dying from cancer (excluding leukemia) increases by 2 percent. For a dose of 100 mSv, that risk increase is at 10 percent. By comparison, risk of dying from cancer was increased by 32 percent for the survivors of the atomic bombing of Hiroshima and Nagasaki.[22]
See also
- Alpha particle
- Beta particle
- Annihilation
- Gamma camera
- Gamma-ray astronomy
- Gamma-ray burst
- Gamma spectroscopy
- Mössbauer effect
- Nuclear fission and fusion
- Radioactive decay
References
- ^ P. Villard (1900) "Sur la réflexion et la réfraction des rayons cathodiques et des rayons déviables du radium," Comptes rendus, vol. 130, pages 1010-1012. See also: P. Villard (1900) "Sur le rayonnement du radium," Comptes rendus, vol. 130, pages 1178-1179.
- ^ L'Annunziata, Michael F. (2007). Radioactivity: introduction and history. Amsterdam, Netherlands: Elsevier BV. pp. 55–58. ISBN 9780444527158.
- ^ Rutherford named γ rays on page 177 of: E. Rutherford (1903) "The magnetic and electric deviation of the easily absorbed rays from radium," Philosophical Magazine, Series 6, vol. 5, no. 26, pages 177-187.
- ^ Aharonian, F.; Akhperjanian, A.; Barrio, J.; Bernlohr, K.; Borst, H.; Bojahr, H.; Bolz, O.; Contreras, J. et al. (2001). "The TeV Energy Spectrum of Markarian 501 Measured with the Stereoscopic Telescope System of HEGRA during 1998 and 1999". The Astrophysical Journal 546 (2): 898. Bibcode 2001ApJ...546..898A. doi:10.1086/318321.
- ^ a b Dendy, P. P.; B. Heaton (1999). Physics for Diagnostic Radiology. USA: CRC Press. p. 12. ISBN 0750305916. http://books.google.com/?id=1BTQvsQIs4wC&pg=PA12.
- ^ Charles Hodgman, Ed. (1961). CRC Handbook of Chemistry and Physics, 44th Ed.. USA: Chemical Rubber Co.. p. 2850.
- ^ Feynman, Richard; Robert Leighton, Matthew Sands (1963). The Feynman Lectures on Physics, Vol.1. USA: Addison-Wesley. pp. 2–5. ISBN 0201021161.
- ^ L'Annunziata, Michael; Mohammad Baradei (2003). Handbook of Radioactivity Analysis. Academic Press. p. 58. ISBN 0124366031. http://books.google.com/?id=b519e10OPT0C&pg=PA58.
- ^ Grupen, Claus; G. Cowan, S. D. Eidelman, T. Stroh (2005). Astroparticle Physics. Springer. p. 109. ISBN 3540253122.
- ^ "CGRO SSC >> EGRET Detection of Gamma Rays from the Moon". Heasarc.gsfc.nasa.gov. 2005-08-01. http://heasarc.gsfc.nasa.gov/docs/cgro/epo/news/gammoon.html. Retrieved 2011-11-08.
- ^ Shaw, R. W.; Young, J. P.; Cooper, S. P.; Webb, O. F. (1999). "Spontaneous Ultraviolet Emission from 233Uranium/229Thorium Samples". Physical Review Letters 82 (6): 1109–1111. Bibcode 1999PhRvL..82.1109S. doi:10.1103/PhysRevLett.82.1109.
- ^ "Gamma-Ray Telescopes & Detectors". NASA GSFC. http://imagine.gsfc.nasa.gov/docs/science/how_l2/gamma_detectors.html. Retrieved 2011-11-22.
- ^ Bock, R. K.; et al (2008-06-27). "Very-High-Energy Gamma Rays from a Distant Quasar: How Transparent Is the Universe?". Science 320 (5884): pp 1752–1754. Bibcode 2008Sci...320.1752M. doi:10.1126/science.1157087. ISSN 0036-8075. PMID 18583607.
- ^ [1] Announcement of first close study of a short gamma-ray burst.
- ^ Rothkamm, K; Löbrich, M (2003). "Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses". Proceedings of the National Academy of Sciences of the United States of America 100 (9): 5057–62. Bibcode 2003PNAS..100.5057R. doi:10.1073/pnas.0830918100. PMC 154297. PMID 12679524. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=154297.
- ^ Department for Environment, Food and Rural Affairs (Defra) UK Key facts about radioactivity, 2003
- ^ United Nations Scientific Committee on the Effects of Atomic Radiation Annex E: Medical radiation exposures – Sources and Effects of Ionizing – 1993, p. 249, New York, UN
- ^ Pattison, J. E.; Hugtenburg, R. P.; Green, S. (2009). "Enhancement of natural background gamma-radiation dose around uranium microparticles in the human body". Journal of the Royal Society Interface 7 (45): 603. doi:10.1098/rsif.2009.0300.
- ^ US National Council on Radiation Protection and Measurements – NCRP Report No. 93 – pp 53–55, 1987. Bethesda, Maryland, USA, NCRP
- ^ "PET/CT total radiation dose calculations. Accessed June 23, 2011." (PDF). http://radiology.rsna.org/content/251/1/166.full.pdf. Retrieved 2011-11-08.
- ^ "Lethal dose", NRC Glossary (October 18, 2011)
- ^ IARC – Cancer risk following low doses of ionizing radiation – a 15-country study – http://www.iarc.fr/ENG/Units/RCAa1.html
External links
- Basic reference on several types of radiation
- Radiation Q & A
- GCSE information
- Radiation information
- Gamma ray bursts
- The Lund/LBNL Nuclear Data Search – Contains information on gamma-ray energies from isotopes.
- Mapping soils with airborne detectors
- The LIVEChart of Nuclides – IAEA with filter on gamma-ray energy, in Java or HTML
- Health Physics Society Public Education Website
|
|||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||||||||