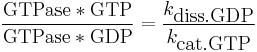GTPase
GTPases (singular GTPase) are a large family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP).[1] The GTP binding and hydrolysis takes place in the highly conserved G domain common to all GTPases.
Contents |
Functions
GTPases play an important role in:
- Signal transduction at the intracellular domain of transmembrane receptors, including recognition of taste, smell and light.
- Protein biosynthesis (aka translation) at the ribosome.
- Control and differentiation during cell division.
- Translocation of proteins through membranes.
- Transport of vesicles within the cell. (GTPases control assembly of vesicle coats).
Mechanism
The hydrolysis of the γ phosphate of GTP into guanosine diphosphate (GDP) and Pi, inorganic phosphate, occurs by the SN2 mechanism (see nucleophilic substitution) via a pentavalent intermediate state and is dependent on the magnesium ion Mg2+.
Major motifs
In most GTPases, the specificity for the base guanine is imparted by the base-recognition motif, which has the consensus sequence [N/T]KXD. [2]
Regulatory GTPases
Regulatory GTPases, also called the GTPase superfamily, are GTPases used for regulation of other biochemical processes. Most prominent among the regulatory GTPases are the G proteins.
GTP switch
All regulatory GTPases have a common mechanism that enables them to switch a signal transduction chain on and off. Toggling the switch is performed by the unidirectional change of the GTPase from the active, GTP-bound form to the inactive, GDP-bound form by hydrolysis of the GTP through intrinsic GTPase-activity, effectively switching the GTPase off. This reaction is initiated by GTPase-activating proteins (GAPs), coming from another signal transduction pathway. It can be reverted (switching the GTPase on again) by Guanine nucleotide exchange factors (GEFs), which cause the GDP to dissociate from the GTPase, leading to its association with a new GTP. This closes the cycle to the active state of the GTPase; the irreversible hydrolysis of the GTP to GDP forces the cycle to run only in one direction. Only the active state of the GTPase can transduce a signal to a reaction chain.
Switch regulation
The efficiency of the signal transduction via a GTPase depends on the ratio of active to inactive GTPase. That equals:
with kdiss.GDP being the dissociation constant of GDP, and kcat.GTP the hydrolysis constant of GTP for the specific GTPase. Both constants can be modified by special regulatory proteins.
The amount of active GTPase can be changed in several ways :
- Acceleration of GDP dissociation by GEFs speeds up the building of active GTPase.
- Inhibition of GDP dissociation by Guanine nucleotide dissociation inhibitors (GDIs) slows down the building of active GTPase.
- Acceleration of GTP hydrolysis by GAPs reduces the amount of active GTPase.
- GTP analogues like γ-S-GTP, β,γ-methylene-GTP, and β,γ-imino-GTP that cannot be hydrolyzed fixate the GTPase in its active state.
Heterotrimeric G proteins
These G proteins are made from three subunits, with the G domain located on the largest one (the α unit); together with the two smaller subunits (β and γ units), they form a tightly associated protein complex. α and γ unit are associated with the membrane by lipid anchors. Heterotrimeric G proteins act as the specific reaction partners of G protein-coupled receptors. The GTPase is normally inactive. Upon receptor activation, the intracellular receptor domain activates the GTPase, which in turn activates other molecules of the signal transduction chain, either via the α unit or the βγ complex. Among the target molecules of the active GTPase are adenylate cyclase and ion channels. The heterotrimeric G proteins can be classified by sequence homology of the α unit into four families:
- Gs family. These G proteins are used in the signal transduction of taste and smell. They always use the activation of adenylate cyclase as the next step in the signal chain. The s stands for stimulation. Their function is permanently activated by the cholera toxin, which is the cause of the fatal effects of infection with Vibrio cholerae.
- Gi family. The i stands for inhibition of the adenylate cyclase; another effector molecule for this protein family is phospholipase C. Also, Gt and Gg proteins are summarized under this label due to sequence homologies. Gt proteins, aka transducin, is used in the light recognition pathway in retina cells. Gg protein occurs in the taste recognition for bitter. Most Gi protein family members can be inhibited by the pertussis toxin of Bordetella pertussis.
- Gq family. These proteins usually have phospholipase C as effector protein.
- G12 family. These G proteins can be activated by thromboxan receptors and thrombin receptors. Their effector proteins are unknown.
By combination of different α, β and γ subunits, a great variety (>1000) G proteins can be produced. GDP is not needed for GTP.
Activation cycle of heterotrimeric G proteins
In the basic state, the Gα-GDP-Gβγ complex and the receptor that can activate it are separately associated with the membrane. On receptor activation, the receptor becomes highly affine for the G protein - GDP complex. On binding with the complex, GDP dissociates from the complex; the receptor works as a GEF - GDP-GTP Exchange Factor; the free complex has a high affinity for GTP. Upon GTP binding, both Gα-GTP and Gβγ separate from both the receptor and from each other. Depending on the lifetime of the active state of the receptor, it can activate more G proteins this way.
Both Gα-GTP and Gβγ can now activate separate and/or the same effector molecules, thus sending the signal further down the signal reaction chain. Once the intrinsic GTPase activity of the α unit has hydrolyzed the GTP to GDP, and then the two parts associate to the original, inactive state. The speed of the hydrolysis reaction works as an internal clock for the length of the signal.
The Ras GTPase superfamily
These are small monomeric proteins homologous to Ras. They are also called small GTPases. Small GTPases have a molecular weight of about 21 kilodaltons and generally serve as molecular switches for a variety of cellular signaling events. According to their primary amino acid sequences and biochemical properties, the Ras superfamily is further divided into five subfamilies: Ras, Rho, Rab, Arf and Ran. The Rho subfamily is further divided into RHOA, RAC1, and CDC42.[3]
Translation factor family
These GTPases play an important role in initiation, elongation and termination of protein biosynthesis.
Translocation factors
See signal recognition particle (SRP).
Large GTPases
See dynamin as a prototype for large GTPases.
See also
References
- ^ Scheffzek K, Ahmadian MR (December 2005). "GTPase activating proteins: structural and functional insights 18 years after discovery". Cellular and Molecular Life Sciences : CMLS 62 (24): 3014–38. doi:10.1007/s00018-005-5136-x. PMID 16314935.
- ^ Leipe D.D., Wolf Y.I., Koonin E.V., and Aravind, L. (2002). "Classification and evolution of P-loop GTPases and related ATPases". J Mol Bio 317 (1): 41–72. doi:10.1006/jmbi.2001.5378. PMID 11916378.
- ^ Hippenstiel S, Schmeck B, N'Guessan PD, et al. (October 2002). "Rho protein inactivation induced apoptosis of cultured human endothelial cells". Am. J. Physiol. Lung Cell Mol. Physiol. 283 (4): L830–8. doi:10.1152/ajplung.00467.2001. PMID 12225960. http://ajplung.physiology.org/cgi/pmidlookup?view=long&pmid=12225960.
External links
- MeSH GTPase
- Neurofibromin 1 from GeneCards
- GTPases: From The Bench (blog)
|
|||||||||||||||||||||||||||||||||||||||||||||||