FLiNaK
FLiNaK is the name of the ternary eutectic alkaline metal fluoride salt mixture LiF-NaF-KF (46.5-11.5-42 mol %).[1] It has a melting point of 454 °C and a boiling point of 1570 °C. It is used as electrolyte for the electroplating of refractory metals and compounds like titanium, tantalum, hafnium, zirconium and their borides. FLiNaK also could see potential use as a coolant in the Very High Temperature Reactor, a type of nuclear reactor.
Contents |
Coolant
FLiNaK salt was researched heavily during the late 1950s by Oak Ridge National Laboratory as potential candidate for a coolant in the Molten Salt Reactor because of its low melting point, high heat capacity, and its chemical stability at high temperatures.[2] Ultimately, its sister salt, FLiBe, was chosen as the solvent salt for the molten salt reactor due to a more desirable nuclear cross section.[3] FLiNaK still gathers interest as a intermediate coolant for a high temperature molten salt reactor where it could transfer heat without being in the presence of the fuel.
Corrosion
Fluoride salts, like all salts, cause corrosion in most metals and alloys. There are three mechanism through which they can cause corrosion: impurities in the salt, temperature gradients in the salt system, and chemical activity gradients.[4] O2, H20, and all oxides are the main impurities that cause corrosion through the following reactions:
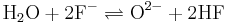
- Water and fluoride ions create acid and oxygen.
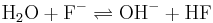
- Water and fluoride ions create acid and hydroxide.
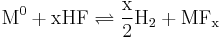
- Acid oxidizes M, where M is any suitable metal (ex: Cr, Fe, Ni).
All of these reactions work together to create hydrofluoric acid which is highly corrosive to many metals. All of these reactions can be reversed by adding a combination of hydrogen gas and hydrofluoric to the molten salt yielding water, which can be boiled off. Theoretically, if contaminates could be taken out of the salt, no corrosion would occur. This process is accelerated by the high temperatures encountered in a nuclear reactor and is one of the main problems in a molten salt reactor. Some compounds, such as the nickel-based Hastelloy-N were engineered specifically for the molten salt reactor experiment and proved highly resistant to corrosion by molten fluoride salts.[5]
See also
References
- ^ http://www.energyfromthorium.com/pdf/FFR_chap12.pdf Lane, James A. Fluid Fuel Reactors. Reading, MA: Addison-Wesley Pub, 1958, p. 570
- ^ http://www.energyfromthorium.com/pdf/FFR_chap12.pdf Lane, James A. "Chemical Aspects of Molten Fluoride Salt Reactor Fuels." Fluid Fuel Reactors. Reading, MA: Addison-Wesley Pub., 1958.
- ^ http://www.energyfromthorium.com/pdf/FFR_chap12.pdf Lane, James A. Fluid Fuel Reactors. Reading, MA: Addison-Wesley Pub, 1958, p. 574
- ^ http://www.inl.gov/technicalpublications/Documents/4502649.pdf Anderson, Mark, Kumar Sridharan, Luke Olson, Paul Brooks, James Ambrosek, Matt Ebner, Piyush Sabharwall, Manohar Sohal, and Phil Sharpe. ``Molten Salts for High Temperature Reactors: University of Wisconsin Molten Salt Corrosion and Flow Loop Experiments - Issues Identified and Path Forward. (2010). Print.
- ^ DeVan, Jackson H. "EFFECT OF ALLOYING ADDITIONS ON CORROSION BEHAVIOR OF NICKEL - MOLYBDENUM ALLOYS IN FUSED FLUORIDE MIXTURES." Thesis. University of Tennessee, 1960. Web. <http://moltensalt.net/references/static/downloads/pdf/ORNL-TM-0202.pdf>.