Electrocommunication
Electrocommunication is the communication method used by weakly electric fishes. Weakly electric fishes are a group of animals that utilizes a communicating channel that is "invisible" to most other animals: electric signaling. Electric fishes communicate in a process called electrocommunication by generating electric fields and interpreting the signal frequencies, waveforms, and delay, etc.[1] The best studied species that conduct electrocommunication are two freshwater lineages- the African Mormyrids and and the South American Gymnotiformes.[2] While weakly electric fish is the only group that has been identified to carry out both generation and reception of electric fields, other species were also identified to perform either ability (but not both). Animals that either generate or receive electric fields are found only in aquatic (or at least moist) environments due to large resistance of all other media (ex. air)[3] So far, communication between electric fish has been identified mainly to serve the purpose of conveying information on
- species recognition
- courtship and sex differentiation
- motivational status (warning and territory protection) and
- environmental resources.
Contents
|
Overview of Weakly Electric Fish
Electric fish is a general term used to describe fish that can generate external electric fields or receive electric fields (electroreception). Electric fish can be further divided into three categories: strongly discharging, weakly discharging, and fish that can only sense but are unable to generate electric fields[1] Strongly electric fish generates strong electric field up to 500 volts for predatory purposes;[4] strongly electric fish includes both marine and fresh water fishes (two freshwater taxa- African electric catfish (Malapterurus electricus) and the Neotropical electric eel (Electrophorus electricus) and the marine torpedo rays (Torpedo)). Weakly electric fish generates electric fields mainly for communicational and electrolocational purposes; weakly electric fish is found in fresh water only and include African freshwater Mormyridae and Gymnarchus and Neotropical electric knifefishes Lastly, fish that are only able to detect electrical signals includes sharks, rays, and skates.[4]
Electric fishes generate discharge from electric organs located near the tail region of the fish. Electric organs are mostly derived from muscle cells (myogenic) with one exception of organs derived from neurons (neurogenic organs) in one gymnotiform family. To detect the electric signals, electric fish has two types of receptive cells- ampullary and tuberous electroreceptors.
Receptive Organs
All organisms respond to sufficiently strong electric shocks, but only some aquatic vertebrates can detect and utilize weak electric field; these organisms are therefore called electroreceptive (For an example, we human-beings react to strong electric currents with a sense of pain and sometimes a mixture of other senses; however, we cannot detect weakly electric fields and therefore are not electroreceptive.) The ability to sense and utilize electric fields was found almost solely in lower, aquatic vertebrates (fishes and some amphibians). Terrestrial animals, with very few exceptions, lack this electric sensing channel due to low conductivity of air, soil, or media other than aqueous environment. Exception in terrestrial vertebrates include Australia monotremes and platypus that sense electric fields generated by fish for hunting.[5]
In order to detect weakly electric fields, animals must possess electroreceptors, or receptive organs, that detect potential differences. For electric fishes, receptive organs are groups of sensory cells rooted in epidermal pits that look like small spots on the skin. In each receptive organ, there are sensory cells embedded in the bottom of the opened "pit" that faces outside. Electroreceptors detect electric signals by building up a potential difference between the outside environment and the fish body's internal environment; current flow due to such potential difference futher results in a receptor potential that’s presynaptic to the sensory fibers. Finally, this receptor potential leads to action potential fired by sensory cells.[6]
Electric fishes carry a variety of sensitive receptive organs that are tuned to different types and ranges of signals. To classify types of electroreceptors, the first branch should be made between ampullary and tuberous organs, which exist in both mormyrids and gymnotiforms. These two types of electric receptors have very distinct anatomical differences- ampullary organs have their opened "pit" formed in a duct-like structure and filled with mucous substance; meanwhile, the "pit" of a tuberous organ is loosely packed with epithelial cells. In addition to anatomical differences, these two receptors also have distinctive functional differences. Ampullary organs are more sensitive and tuned to a low frequency range of 1-10Hz, which is the range of non-electrogenic, biological source of electricity; therefore, ampullary organs are mainly used for passive electrolocation. On the other hand, tuberous, which are used for electrocommunication by weakly electric fishes, are less sensitive and tuned to much higher frequencies.[6][7]
Classification of the two types of receptive organs
(Modified from Carl D. Hopkins' website on Electroreception)
| Type | Structure | Function | Sensitivity | Where found |
|---|---|---|---|---|
| Ampullary | Open pit/ Filled with mucous | Electrolocation/ Locate preys | High: 0.01 μV/cm in marine species, 0.01 mV/cm in freshwater; Sensitive to DC fields/ Low frequencies less than 50 Hz | Sharks & Rays; Non-teleost fishes; Certain teleosts (mormyrids, certain notopterus, gymnotiforms, catfish); Amphibians (except frogs and toads) |
| Tuberous | Covered by skin - loosely packed with epithelia cells | Electrocommunication | 0.1 mV to 10 mV/cm/ Tens of Hz to more than 1 kHz. | Mormyrid fish; Gymnotiform fish |
Tuberous organs
Tuberous organs, the type of receptive organ used for electrocommunication, can be divided into two types depending on the way information is encoded: time coders and amplitude coders. There are multiple forms of each type of tuberous organs, and all weakly electric fish species possess at least one form of both time coders and amplitude coders. Time coder fires phase-locked action potential (meaning, the waveform of the action potential is always the same) at a fixed delayed time after each outside transient is formed. Therefore, time coders neglect information about waveform and amplitude, but focus on frequency of the signal and fire action potential on a 1:1 basis. Amplitude coders, on the contrary, fire according to the EOD amplitude. While both wave-type and pulse-type fishes have amplitude coders, they fire in a different way: receptors of wave-type fishes continuously fire at a rate according to their EOD amplitude; receptors of pulse-type fishes fire burst of spikes to each EOD detected and the number of spikes in each burst is correlated to the amplitude of the EOD. Tuberous electroreceptors show a V-shaped threshold tuning curve (similar to auditory system), which means they are tuned to a particular frequency; the frequency they are tuned to is usually closely matched to their own EOD frequency.[8]
Classification of Tuberous Organs
| Type | Fire according to | Method of encoding | Found in |
|---|---|---|---|
| Time Coder | Frequency of received EOD | Fires action potential in a 1:1 ratio to the received EOD | Both types of weakly electric fishes |
| Amplitude Coder | Amplitude of received EOD | Wave-tyep: continuously fire at a rate according to EOD amplitude/ Pulse-type: number of bursts in each spike is depend on the amplitude of EOD | Both types of weakly electric fishes |
Electric organs
Weakly electric fish generates Electric Organ Discharge (EOD) with specialized compartments called electric organ. Almost all the weakly electric fishes have electric organs derived from muscle cells (myogenic) ; the only exception is the Apteronotidae, a family under Gymnotiforms, that has electric organs derived from nerve cells (neurogenic). Myogenic electrocytes are arranged into columns of small, disks-like cells called electroplate. Apteronotidae, being the exception, also carries myogenic electric organs in larval stages; however, as the fish matures, electrogenic organs derived from central spinal cord gradually replace the muscle cell-derived electric cells.[9]
Discharge of an electric organ begins with central command from a medullary pacemaker that determines the frequency and rhythm of EODs, which is also referred to as SPI- sequence of pulse intervlas; the command is then passed on by spinal electromotor neurons to electrocytes that form the electric organ, which determine the waveform of the EODs based on its morphophysiological properties. As commands from pacemaker reaches electric organ, it opens up all the sodium channels, causes net sodium ion flux in one direction (either towards or away from the head, and brings simultaneous depolarization of all electroycytes on the same side of cell. The result is a positive polarity at the fish's head relative to the tail, or vice versa: a dipole system. The polarity built up by the electric organ therefore sets up electrostatic fields in the water.[4][10]
Electric organs are quite different between mormyrids and gymnotiforms and therefore will be presented separately:
Mormyrids
In mormyrids, the electric organ is fairly small and located only in the caudal peduncle region (the narrow part of a fish's body where the caudal fin is attached to). Electric organ is formed by disk-like electrocytes serially connected together in two columns, and each column resides on one side of the spinal cord. The myogenic electrocytes are identical to each other and arranged in a homogenous pattern, which results in synchronized discharge- electrical potential recorded from a single electrocyte is equivalent to the miniature version a complete EOD measured outside of the fish. Electrocytes also have an important structure called "stalk," which are tentacle or tube-like structures that extend out from each electrocytes. Different stalk-electrocyte systems have been observed, which include stalks that penetrate the elctrocytes, innervate electrocytes from the posterior or anterior, and so on. Multiple stacks from one electrocyte eventually fuse together to form a large stalk that receives innervations from spinal electromotor neurons. Different morphological structures of the stalk/ electrocyte system result in differences in electric current flow, which further leads to the various waveforms.[4][8][11]
Gymnotiforms
In gymnotiforms, electrocytes are quite different between wave-type and pulse-type electric fishes. In wave-type fishes, electrocytes are oriented in longitudinal direction in a tubular form. The electocytes also form columns, but unlike the shorter size of electric organ in mormyrids, gymnotiforms have long electric organs that extend through almost the entire longitudinal body length. Also different from the multiple ways stalk system innervates the electrocytes in mormyrids, stalks in gymnotiforms only make one type of innervations at the posterior side of electrocyte. Pulse-type gymnotifoms generally show a higher complexity than the wave-type fishes. For instance, their electrocytes can be either cylindrical or drum-shaped with stalks innervating from either posterior or anterior side. Another important difference is that, unlike mormyrids or wave-type gymnotiforms, electrocytes of pulse-type gymnotiforms are not homogenous along the long electric organ that traverses the fish body. The in-homogeneity of electrocytes leads to EODs with highly variable and complex spatial-temporal properties.
Apternotids, being a member of the wave-type gymnotiforms, is different from all other electric fishes by possessing the only neurogenic electrocytes. Electric organ of Apternotids is derived from neurons; more specifically, they are formed from the axons of spinal electromotor neurons. Such structure eliminates one [synapse|synaptic gap] between spinal electromotor neuron and the myogenic electrocytes, which might contribute to Apternotids' highest EOD frequency (>1000Hz) among electric fishes.[8]
Signals
Types of Signals
There are two types of signals generated by electric fishes: pulse-types and wave-types, and each electric fish produces only one of the two distinct types of signals. A pulse-type EOD is characterized by discrete EOD fires separated by silent intervals much longer than the discharges; contrarily, a wave-type EOD has its firing period and silence period approximately the same in length, and therefore a continuous signal with quasi-sinusoidal waveform is formed. Among the two mostly-studied groups of weakly electric fish, the mormyrids and gymnotiforms, both pulse-type and wave-type fishes are found and are grouped by families.[12]
Physical Properties of Signals
Electric Field
Electric fish generates signals by forming an electric field from a dipole-like system made by their electric organ. The electric field from a dipole forms closed field lines pointing from the positive pole to the negative (can be either head or tail), which makes the electric fish EOD vastly different from other communicating modes that have propagating waves. While sound waves for acoustic communication, chemical gradients for chemical communications, or light waves (electromagnetic waves) for visual communications all propagate, electric signals do not (it is different from electromagnetic waves). As an electric field, the signal magnitude decreases as the inverse cube of distance (1/(r^3)), which makes the signal sending and formation a energy-costly process. To solve this problem, electric fish matches the impedance of their electric organ to the conductivity of water in order to achieve minimum energy lost, and the final result is electric signals traveling for at most a few meters. Although electric fish signal has its limit on traveling distance, it does not deteriorate with factors that affect waves, which include reflection, refraction, absorption, interference, and so on. As a result, the temporal features, which are very important for electric fish signals, strictly remain constant in space.[13]
Active Space
When transmitting electric signals in aquatic environment, the physical and chemical nature of the surrounding can make big differences on signal transmission. Environmental factors that might impose influences include solute concentration, temperature, and background electric noise (lightning or artificial facilities), etc. To understand the effectiveness of electric signal transmission, it is necessary to define the term "active space-" the area/ volume within which a signal can elicit responses from other organisms. The active space of an electric fish normally has an ellipsoid shape due to the arrangement of dipoles formed by its electric organs. While both electric communication and electrolocation rely on signals generated by electric organs, electrocommunication has an active space tenfold larger than electrolocation because of the extreme sensitivity of tuberous electrocommunication receptors. [14]
One of the biggest factors that affect active space size will be the [water conductance] mediated by solute concentration in the water. It’s been shown that momyrids have adapted its optimal active range in lower-conductivity habitats. One natural phenomenon that supports such theory is that, many species spawn during the time when rivers/ lakes have the lowest conductivity due to heavy rains. Having bigger active space in water with low conductivity will therefore benefit mating and courting.[15] One other explanation tested by Kim and Moller is that, having smaller active space during dry season when mating is not occurring accommodates crowded social spacing without unnecessary signal transmission between individuals.[4]
Frequency and Waveform
Electric fishes communicate with electric signals that possess two main qualities- frequency and waveform. The information in waveform is embedded in the electric organ discharge (EOD) itself, which is determined and fixed by the anatomy and physiology of the electric organ. EOD waveform, in some species, changes with developmental stages. Frequency of EODs and interval duration between them are called sequence of pulse intervals (SPI), which is controlled by the command interneurons in the midbrain and medulla, as stated under electric organs. Alteration in SPI produces widely varying social signals among electric fish during mating, warning, or identifying. These two properties (waveform/EOD and frequency/SPI) are used by both wave and pulse type fishes for recognition and communication.[16]
EOD Frequency
Frequency is the number of occurrences of a repeating event per unit time. Here, EOD frequency is referred to the firing rate pf an electric fish. Wave-type fishes carry out species recognition by mediating their EOD frequencies, which include their baseline firing frequencies and modulation of frequencies that results in rising, falling, warbling, and cessations of EOD frequencies. For an example, some gymnotiform species use "chirps," a sudden frequency increase, during courtship.
EOD Waveform
Waveform is the shape and form of a wave. Each species of electric fish has their distinct EOD waveform. Pulse-type fishes conduct species recognition by paying attention to the differences of EOD waveform, which include properties such as: EOD duration, number of phases, and form of the phases. Meanwhile, some indirect properties hidden in waveform are also used by pulse-type fishes: amplitude gradient, duration ratios of phases, and the order and signs of phases.
Differences and Changes in Signals
Electric fishes normally possess a baseline frequency and waveform of their signals, alteration in both qualities occur all the time- among different species, sex, development stages, and dominance status. While different alterations occur to signal generations based on the fishes' identities, the level and types of alteration is limited by the fishes' own sensory system, which is biased to sense signals that have similar frequency to its own discharge frequency.[6]
Signals and Sex
As electric fish matures, some taxa develop differences in EOD between male and female (sexual dimorphism). Typically, male electric fish has lower EOD frequency and longer EOD duration than female; among males, the dominated and biggest fish generally possesses the lowest frequency. For an example, as measurements made in Sternopygus marucus (Hagedorn, 1986) showed, males usually generates EOD at about 80Hz, while females generate EOD at around 150Hz. Such differences in EOD between the two sexes can be traced back to the changes in the action potential in electrocytes. As electric fish matures from juvenile stage, male fish grows larger with longer and thicker tail, which might result in larger electric organ that generates lower frequency EODs.[17][18] One of the physiological factors that have been proven to contribute to the sexual dimorphism of EODs is the level of teleost hormone- androgen 11-keto-testosterone (11-KT) and estrogen. Experiments shown that by injecting 11-KT to female electric fishes, not only did their EOD waveforms and frequencies turn closer to that of the males, but their tuberous electroreceptors were also modified to be able to detect signals according to the newly-transformed EOD properties. On the other hand, when estrogen was applied, male electric fishes’ EODs gradually turn closer to the female EODs.[6][19]
Sexual dimorphism in EOD waveforms and frequencies also imposes an influence on active space size. Using species Sternopygus marucus as example, male and female emits frequencies almost twice as different from each other (80 Hz vs 150 Hz). However, since most electroreceptors are tuned to signal frequencies that are closer to the receivers' own frequency, the difference in EOD frequency results in different ability of electric fishes to sense signals from either sex, which further leads to different active space sizes. As measured in Sternopygus marucus by Hagedorn, male fish can only detect female in a range of 6cm, while female fish can detect a male fish in a much bigger range of 39cm. This active space size difference is hypothesized to give female fishes a better chance to get closer to potential mates and select the right individual to mate with.[18][4][17]
Signals and Development Stages
Studies done on both gymnotiforms and mormyrids have shown that there are species in both groups that have significant EOD changes from larvae to adult. Gymnotiform larvae all have EODs that are simple, monophasicaly similar to a single period cosine function, and formed with a very broad spectrum at lower frequency range. It is observed that, as the larvae mature, the frequency spectrum decreases, the discharge waveform becomes sharper, and more complex waveforms that may consist of multiple phases gradually replace the simple larval EOD.[20]
For the myogoenic fishes, this change in signal waveform occurs with the initial larval electrocytes fusing together forming new electrocytes with different shapes, along with redistribution of ion-gated channels, formation of new extracellular structures on the electrocytes, etc. Some pulse fishes also develop accessory electric organs located on other parts of the body; these extra electric organs further play a role in adding phases to the EODs. For the only neurogenic fish known so far, apteronotids, EOD changes during the developmental process seemed to be more dramatic than that of the myogenic fishes, which might indicate that neurogenic electrocytes are more easily prone to modifications. Similar with the myogenic fishes, apteronotids has its electric organ formed by myocytes. As apteronotids matures, new neurogenic electrocytes derived from spinal motoneurons replace the myogenic electrocytes.[9]
There were two hypotheses proposed for the reason why electric signals were modified during the fishes' development stages. Firstly, as stated above, the fishes' electroreceptors are usually tuned to a specific range of frequencies. Therefore, to make effective communication, it is necessary for the electric fishes to narrow the broad frequency spectrum of the larval EOD. Secondly, it is known that the electroreceptors of catfish, gymnotiforms, and most pre-teleost fish are tuned to lower frequencies. Therefore, keeping the low frequency of larval EOD will increase the risk of being detected by predators.[6]
Signals and dominance status
Measurements have shown that typically, male electric fish that’s dominating usually has lower EOD frequency and longer EOD duration. An experiment has shown that, when two males are placed in the same fish tank, both fishes enhance their EOD in the first short period of time. However, after leaving the fishes in a dark period (mimicking night time), the male with higher EOD amplitude, which is also usually the male with bigger body size, will further enhance its EOD; on the contrary, the male with smaller body size/ smaller EOD does not enhance its EOD.[21]
Special Signals
In electric communication, there are some distinct types of signals that serve special purposes such as courting or aggression. Examples of these special EODs include: "rasps", "chirps" and "smooth acceleration". Rasp is a burst of pulses at a relatively constant frequency performed by some species during courtship. Chirp is a rapid increase or decrease in frequency. Smooth acceleration is a period of tens to hundreds of milliseconds that EOD rate increases but in a smooth way. Due to law of conservation of energy, the amplitude of the EOD might lower for a few percent, but the overall changes in waveform and amplitude Is small. Male gymnotiforms emit these accelerated signals during aggression and courtship. In the fish studied, if courtship goes well and proceeds to spawning, male electric fish starts to use another special type of EOD- the chirp. Chirp also lasts for tens to hundreds milliseconds; however, the increase in frequency was so high that electrocytes could not recover soon enough, and therefore, chirps has a very small amplitude and a waveform deviated from the original waveform.[22][16]
Measuring Signals
Quantitative measures
Quantitatively measuring and visualizing electric signals could mean great challenge and required equipments including electrodes, analog-to-digital converters, Fourier transforms, Delaunay tessalations, and so on. Measurements and processed-data include electric potential gradients, electric field maps and discharge waveforms/ frequencies. Complex computer simulations, computing, and modeling are also required to further examine the signals.[6]
Coding electric signals
In general, neural coding is a process in which information from sensory stimulus, which include light, pressure, and sound, etc., is coded into a "language" the brain can understand; these new codes are then represented to the brain by networks of neurons. External stimuli differ on their duration, intensity, form, direction, and more; to represent these differences in sensory stimuli, sensory neurons change their action potential firing in various temporal patterns- sequences, duration, or frequencies.[23][24]
For electric fishes, EOD coding is the process in which electric fishes code external electric signals, which vary in waveform and frequencies, into patterns of action potential firing and present it to the brain. There are two main EOD coding schemes that have been studied: rate coding and temporal coding. Rate coding focuses simply on the various "firing rate." Temporal code, on the other hand, focuses on the precise timing of single spikes, the timing of rise and falls in a EOD (waveform), etc.[19]
Frequency Coding
Rate coding in the field of neural coding has been the earlier and traditional coding scheme, which assumes that information is only carried in the rate of firings. Due to this reason and our understanding in action potential's all-or-none properties, it was natural that the researches in EOD coding started with frequency coding. Generally, frequency coding is established on the following principles:
- Electric fish has their own firing frequency range
- Frequency normally increases with stronger stimuli
- Combined frequency is used to communication in some wave-type fishes.
Wave-type electric fish uses frequency coding during electrocommunication. For one thing, since wave-type fishes constantly generates a continuous signal, they need a strategy to distinguish self and neighbor signals. For instance, in order to prevent interference between two signals, when two neighboring fishes generate signals that have frequency too close to each other, both fish temporarily shifts its frequency away from the original point- the well-studied Jamming Avoidance Respond.[25] Before the actual circuitry and neurophysiological properties were solved, there were several behavior experiments done on wave-type fishes that proved the usage of frequency coding:
- When playback of artificially generated sine-waves with different frequencies were presented to males, they show greater number of attacks and chirps when the frequency is within their own range of EOD frequency.
- When playback of male chirps within the conspecific frequency to females, they produce spawning behavior.[6][26]
Combined frequency
Following the behavior experiments, further researches have shown that wave-type electric fishes utilize characteristic of combined-waves to identify species, sex, and other status of the other fishes. Since wave-type fishes are constantly firing and have no corollary discharge, their tuberous organs receive electric signals from other fishes and their own EOD at the same time. When two signals with very close frequencies (difference < 5Hz) comes in, due to the physical properties of waves, the two waves will “combine” and form two new sinusoidal waves from the modulation of both frequency and amplitude, which requires the participation of both amplitude-coding and time-coding receptors.
Amplitude modulation results in "beats"- a new sinusoidal wave with its frequency equal to the frequency difference of the original two waves (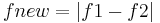 ). Amplitude-coding units read the beats and fire synchronically with the beats. Therefore, amplitude-coding units provide information on the difference in frequency (∆f) but cannot determine the "sign," which essentially means "which fish has higher EOD frequency." To determine the sign of ∆f, the fishes need to compare information from both amplitude and frequency modulation. Frequency modulation of the two signals results in new sinusoidal wave that has firing times alternate between lagging and leading phases. It is discovered that with different sign of ∆f, the relation between phase and amplitude modulation is opposite. (When ∆f > 0, phase lags while amplitude increase and leads while amplitude decrease; when ∆f < 0, phase lags when amplitude decreases and leads when amplitude increases).Therefore, by integrating information from two sources (self and another sender) and then combining information from two modulations, wave-type fish is able to determine the EOD frequency of another fish and determine the identity of others.
). Amplitude-coding units read the beats and fire synchronically with the beats. Therefore, amplitude-coding units provide information on the difference in frequency (∆f) but cannot determine the "sign," which essentially means "which fish has higher EOD frequency." To determine the sign of ∆f, the fishes need to compare information from both amplitude and frequency modulation. Frequency modulation of the two signals results in new sinusoidal wave that has firing times alternate between lagging and leading phases. It is discovered that with different sign of ∆f, the relation between phase and amplitude modulation is opposite. (When ∆f > 0, phase lags while amplitude increase and leads while amplitude decrease; when ∆f < 0, phase lags when amplitude decreases and leads when amplitude increases).Therefore, by integrating information from two sources (self and another sender) and then combining information from two modulations, wave-type fish is able to determine the EOD frequency of another fish and determine the identity of others.
Temporal Coding
Temporal coding was first being neglected by researchers studying electric fish signals because:
- EOD seemed to be too short to include information within
- The idea of communicating with signals coded in the same way of action potential was easier to be accepted
Therefore, research on signal coding was first focused on SPI (sequence of pulse intervals) until playback experiments were done in the history to prove that, temporal coding fishes (pulse-type) focus on EOD itself but not SPI. Utilizing male fish courting calls of rasps (highly rapid discharge), experiments done by Hopkins that proved the low importance of SPI in pulse-type fish include:
- When playback of sine wave with conspecific female SPI was presented to males, no response was generated from the male (which proved that frequency was not the code)
- Male fishes were presented with playback of 1) original female EOD; 2) phase-shifted female EOD; 3) time reversed female EOD- No response in the phase-shifted group but rasping behavior was observed in the other two playbacks
- Male fishes were presented with computer generated signals that have the following options mixed up in all possible combinations: male/ female, conspecific/ not-conspecific EOD, and conspecific/ not-conspecific SPI- It was observed that to elicit rasps, the combination must include: female, conspecific EOD, and lastly, either types of SPI
These experiments pointed to a coding scheme that pays attention to EOD itself (waveform, amplitude gradient, duration ratios of phases, etc.) but not the the frequency. Further researches found out the receptor that is responsible for temporal coding: the knollenorgan receptors. When the sender builds up an electric field during communication, electric current flows into the receiver body from one side and exit from the other. Therefore, between electric receptors on each side, the direction of electric current flow is opposite and results in opposite stimulus polarities to the receptor. Electric physiology recording shows that under normal polarity (positive to negative, which occurs on the side where electric current enters the fish body), knollenorgans fire to the onset of a current; on the contrary, under reversed polarity (negative to positive, which occurs on the side where electric current leaves the fish body), knollenorgans fire to the offset of a current. By comparing the inputs from knollenorgans on each side of the fish, a pulse-type electric fish can determine information of the signal that includes waveform, wave duration, and so on.[27][28][4]
References
- ^ a b Masashi Kawasaki. The electric fish. [1] Retrieved 12/3/2011
- ^ Map of Life, 2011 [2]
- ^ Nicole U. Czech-Damal, Alexander Liebschner, Lars Miersch, Gertrud Klauer, Frederike D. Hanke, Christopher Marshall, Guido Dehnhardt, and Wolfz Hanke (2011). [3] Proc Biol Sci. doi:10.1098/rspb.2011.1127.
- ^ a b c d e f g Moller, P. (1995) Electric Fishes: History and Behavior. Chapman & Hall
- ^ Electroreception and Communication in Fishes / Bernd Kramer - Stuttgart; Jena ;Lübeck ; Ulm : G. Fischer, 1996. Progress in Zoology ; Vol. 42.
- ^ a b c d e f g Ladich, Friedrich. 2006. Communication in fishes. Enfield, NH: Science Publishers
- ^ Carl Hopkins. Electroreception. [4] Retrieved 12/5/2011
- ^ a b c Masashi Kawasaki Chapter 7: Physiology of Tuberous Electrosensory Systems. In: Theodore H. Bullock, Carl D. Hopkins, Arthur N. Popper and Richard R. Fay. 2005 (eds), Electroreception. New York: Springer.
- ^ a b Bennett MVL (1971) Electric organs. In: Hoar WS, Randall DJ (eds), Fish Physiology. London: Academic Press
- ^ Philip K. Stoddard, Electric Signals and Electric Fishes. 2009. [5]
- ^ Hopkins, CD, Design features for electric communication Journal of experimental biology Vol. 202, 10, 1999, p. 1217
- ^ Stoddard PK. (2002) Electric signals: predation, sex, and environmental constraints. Advances in the Study of Behaviour
- ^ Hopkins, CD, Temporal structure of non-propagated electric communication signals brain behavior and evolution Vol. 28, 1986, p. 43 [6]
- ^ Bossert, WH, The analysis of olfactory communication among animals. Journal of Theoretical Biology. Vol. 5, 3, 1963, p. 443
- ^ Squire, A; Moller, P. Effects of water conductivity on electrocommunication in the weak-electric fish Brienomyrus niger (Mormyriformes)animal behaviourVol. 30, 2, 1982, p. 375
- ^ a b Hopkins, C.D., Neuroethology of electric communicationannual review of neuroscienceVol. 11, 1, 1988, p. 497
- ^ a b Hopkins, C. D. (1972). Sex differences in electric signaling in anelectric fish. Science 176
- ^ a b Hagedorm, M.(1986) The ecology, courtship and mating of gymnotiform electric fish. (eds. Bullock, T. H.Heiligenberg, W.), Wiley, NY
- ^ a b Bass, A. H.; Hopkins, C.D., Shifts in frequency tuning of electroreceptors in androgen-treated mormyrid fish, Journal of comparative physiology, Volume 155, Number 6, 713-724, DOI: 10.1007/BF00611588
- ^ Emergence and development of the electric organ discharge in the mormyrid fish, Pollimyrus isidori, G. W. Max Westby and Frank Kirschbaum, JOURNAL OF COMPARATIVE PHYSIOLOGY A: NEUROETHOLOGY, SENSORY, NEURAL, AND BEHAVIORAL PHYSIOLOGYVolume 122, Number 2, 251-271, DOI: 10.1007/BF00611894
- ^ Franchina,C.R.; Salazar, V.L.; Volmar, C. H, and Stoddard, P.K., Plasticity of the electric organ discharge waveform of male Brachyhypopomus pinnicaudatus. II. Social effects journal of comparative physiology b biochemical systemic and environmental physiology Vol. 187, 1, 2001, p. 45
- ^ Carl Hopkins, Behavioral Evidence for Species Recognition[7], Retrieved 12/6/2011
- ^ Spike arrival times: A highly efficient coding scheme for neural networks, SJ Thorpe - Parallel processing in neural systems, 1990[cerco.ups-tlse.fr/2011/pdf0609/thorpe_sj_90_91.pd]
- ^ Gerstner. 1997. Neural codes: Firing rates and beyond. Proceedings of the National Academy of Sciences of the United States of America 94:12740-12741
- ^ Bullock, T.H., Hamstra R.H., and Scheich H., The jamming avoidance response of high frequency electric fish, Journal of comparative physiology, Biochemical systemic and environmental physiology, Vol. 77, 1, 1972
- ^ Carl D. Hopkins, Electric Communication: Functions in the Social Behavior of Eigenmannia virescens Behavior Vol. 50, No. 3/4 (1974), pp. 270-305 [8]
- ^ Bass, AH; Hopkins, CD. Shifts in frequency tuning of electroreceptors in androgen-treated mormyrid fish journal of comparative physiology b biochemical systemic and environmental physiology Vol. 155, 6, 1984, p. 713
- ^ Neuroethology of species recognition in electroreception . Advances in Vertebrate Neuroethology. New York, Plenum. Hopkins, C. D., Ed. (1983)
External links
- A video of courtship and pawning behavior between a male and female Brienomyrus brachyistius can be seen here.
- Methods for listening to electric fishes at home.