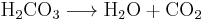Carbonation
Carbonation is the process of dissolving carbon dioxide in water. The process usually involves carbon dioxide under high pressure. When the pressure is reduced, the carbon dioxide is released from the solution as small bubbles, which cause the solution to "fizz." This effect is seen in carbonated soft drinks.
Carbonation can also describe a chemical reaction, one example of which is a key step in photosynthesis.
Contents |
Chemistry
Carbon dioxide is weakly soluble in water, therefore it separates into a gas. The process of carbon dioxide effervescing from a solution is represented by the following chemical reaction, in which aqueous carbonic acid converts to carbon dioxide and water:
Biochemistry
Carbonation also describes the incorporation of carbon dioxide into chemical compounds. Our carbon-based life originates from a carbonation reaction that is most often catalysed by the enzyme RuBisCO. So important is this carbonation process that a significant fraction of leaf mass consists of this carbonating enzyme.[1]
See also
References
- ^ Stryer, Lubert; Berg, JeremyMark; Tymoczko, John L. Biochemistry, 5th Ed. W.H. Freeman, San Francisco, 2002. ISBN 0-7167-3051-0
External links
- Carbonation and Acidity
- Dissolution of Marble in Hydrochloric Acid Demonstration (instruction and video)
- Whirlpools in a soda pop Explains why a shaken soda bottle will spray soda when opened.
- Robert O'Leary. "ATR Infrared Spectroscopy method for measuring CO2 concentration in Beer". http://vitalsensorstech.com/PDF%27s/ATR%20spectroscopy%20method%20for%20dissolved%20CO2%20in%20beverages.pdf. Describes in detail the theory and practice of measuring dissolved CO2 content in soft drinks and beer.