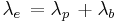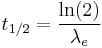Effective half-life
Effective half-life denotes the halving of radioactive material in a living organism by means of radioactive decay and biological excretion. A decay constant is needed to calculate the half-life. It is the sum of the biological and physical decay constants, as in the formula:
With the decay constant it is possible to calculate the effective half-life using the formula:
The biological decay constant is often approximated as it is more difficult to accurately determine than the physical decay constant.
Alternatively, since the radioactive decay contributes to the "physical (i.e. radioactive)" half-life, while the metabolic elimination processes determines the "biological" half-life of the radionuclide, the two act as parallel paths for elimination of the radioactivity, and in analogy with parallel resistors, the effective half-life could also be calculated by the formula[1]:
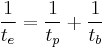 , or
, or
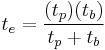 .
.
External links
References
- ^ Biological Effects of Radiation ©1996, Kenneth R. Koehler. All Rights Reserved.