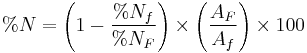Diatomaceous earth
Diatomaceous earth ( /ˌdaɪ.ətəˌmeɪʃəs ˈɜrθ/) also known as diatomite or kieselgur/kieselguhr, is a naturally occurring, soft, siliceous sedimentary rock that is easily crumbled into a fine white to off-white powder. It has a particle size ranging from less than 1 micrometre to more than 1 millimetre, but typically 10 to 200 micrometres.[1] This powder has an abrasive feel, similar to pumice powder, and is very light, due to its high porosity. The typical chemical composition of oven dried diatomaceous earth is 80 to 90% silica, with 2 to 4% alumina (attributed mostly to clay minerals) and 0.5 to 2% iron oxide.[1]
Diatomaceous earth consists of fossilized remains of diatoms, a type of hard-shelled algae. It is used as a filtration aid, mild abrasive, mechanical insecticide,[2] absorbent for liquids, matting agent for coatings, reinforcing filler in plastics and rubber, anti-block in plastic films, porous support for chemical catalysts, cat litter, activator in blood clotting studies, and a stabilizing component of dynamite. As it is also heat-resistant, it can be used as a thermal insulator.
Contents |
Geology and occurrence
Formation
Diatomite forms by the accumulation of the amorphous silica (opal, SiO2·nH2O) remains of dead diatoms (microscopic single-celled algae) in lacustrine or marine sediments. The fossil remains consist of a pair of symmetrical shells or frustules.[1]
Discovery
In 1836 or 1837, the peasant and goods waggoner, Peter Kasten,[3] discovered diatomaceous earth (German: kieselgur) when sinking a well on the northern slopes of the Haußelberg hill, in the Lüneburg Heath in north Germany. Initially, it was thought that limestone had been found, which could be used as fertiliser. Alfred Nobel used the properties of diatomaceous earth in the manufacture of dynamite. The Celle engineer, Wilhelm Berkefeld, recognised its ability to filter, and developed 'filter candles' fired from diatomaceous earth.[4] During the cholera epidemic in Hamburg in 1892, these Berkefeld filters were used successfully.
Extraction and storage sites in the Lüneburg Heath
- Neuohe - extraction from 1863 to 1994
- Wiechel from 1871 to 1978
- Hützel from 1876 to 1969
- Hösseringen from ca. 1880 to 1894
- Hammerstorf from ca. 1880 to 1920
- Oberohe from 1884 to 1970
- Schmarbeck from 1896 to ca. 1925
- Steinbeck from 1897 to 1928
- Breloh from 1907 to 1975
- Schwindebeck from 1913 to 1975
- Hetendorf from 1970 to 1994
The deposits are up to 28 metres (92 ft) thick and are all of freshwater kieselgur.
Until the First World War almost the entire worldwide production of kieselgur was from this region.
Other deposits
In Germany kieselgur was also extracted at Altenschlirf [5] on the Vogelsberg (Upper Hesse) and at Klieken [6] (Saxony-Anhalt).
There is a layer of kieselgur up to 4 metres (13 ft) thick in the nature reserve of Soos in the Czech Republic.
In Colorado and in Clark, Nevada (USA), there are deposits that are up to several hundred metres thick in places.
Sometimes kieselgur is found on the surface in deserts. Research has shown that the erosion of kieselgur in such areas (such as the Bodélé Depression in the Sahara) is one of the most important sources of climate-affecting dust in the atmosphere.
The commercial deposits of diatomite are restricted to Tertiary or Quaternary periods. Older deposits from as early as the Cretaceous Period are known, but are of low quality.[7] Marine deposits have been worked in the Sisquoc Formation in Santa Barbara County, California near Lompoc and along the Southern California coast. Additional marine deposits have been worked in Maryland, Virginia, Algeria and the MoClay of Denmark. Fresh water lake deposits occur in Nevada, Oregon, Washington and California. Lake deposits also occur in interglacial lakes in the eastern US and Canada and in Europe in Germany, France, Denmark and the Czech Republic. The worldwide association of diatomite deposits and volcanic deposits suggests that the availability of silica from volcanic ash may be needed for thick diatomite deposits.[7]
Applications
Industrial
In 1866, Alfred Nobel discovered that nitroglycerin could be made much more stable if absorbed in diatomite. This allows much safer transport and handling than nitroglycerin in its raw form. He patented this mixture as dynamite in 1867, and the mixture is also referred to as guhr dynamite.
Filtration
The most common use (68%) of diatomaceous earth is as a filter medium, especially for swimming pools. It has a high porosity, because it is composed of microscopically-small, coffin-like, hollow particles. Diatomaceous earth (sometimes referred to by trademarked brand names such as Celite and Celatom) is used in chemistry as a filtration aid, to filter very fine particles that would otherwise pass through or clog filter paper. It is also used to filter water, particularly in the drinking water treatment process and in fish tanks, and other liquids, such as beer and wine. It can also filter syrups, sugar, and honey without removing or altering the color, taste, or nutritional properties of any of them.[8] Other industries such as paper, paints, ceramics, soap and detergents use it as a fulling material.
Abrasive
The oldest use of diatomite is as a very mild abrasive and, for this purpose, it has been used both in toothpaste and in metal polishes, as well as in some facial scrubs.
Pest control
Diatomite is used as an insecticide, due to its physico-sorptive properties. The fine powder absorbs lipids from the waxy outer layer of insects' exoskeletons, causing them to dehydrate.[9] Arthropods die as a result of the water pressure deficiency, based on Fick's law of diffusion. This also works against gastropods and is commonly employed in gardening to defeat slugs. However, since slugs inhabit humid environments, efficacy is very low. It is sometimes mixed with an attractant or other additives to increase its effectiveness. Medical-grade diatomite is sometimes used to de-worm both animals and humans, with questionable efficacy.[10][11] It is most commonly used in lieu of boric acid, and can be used to help control and eventually eliminate cockroach and flea infestations. This material has wide application for insect control in grain storage.[12] It has also been used to control bedbug infestations, but this method may take weeks to work.[13]
Absorbent
Its absorbent qualities make it useful for spill clean-up and the U.S. Centers for Disease Control recommends it to clean up toxic liquid spills. These qualities also lend themselves to use in facial masks to absorb excess oils.
It has been employed as a primary ingredient in a type of cat litter. The type of silica used in cat litter comes from freshwater sources and does not pose a significant health risk to pets or humans.
Thermal
Its thermal properties enable it to be used as the barrier material in some fire resistant safes. It is also used in evacuated powder insulation for use with cryogenics.[14] Diatomaceous earth powder is inserted into the vacuum space to aid in the effectiveness of vacuum insulation.
DNA purification
Diatomite (Celite) can be used for the removal of DNA in the presence of a highly concentrated chaotropic agent such as sodium iodide, guanidinium hydrochloride and guanidinium thiocyanate. As with other silicates, the diatomites will remove double stranded DNA but not RNA or proteins. The DNA can be extracted from the diatomites using low ionic strength buffers, including water, at neutral to slightly alkaline pH. Crude diatomites of a uniform size must first be washed in a heated acid such as 5M HCl.[15] Calcination can further improve consistency of the material, while mild caustic treatment may improve adsorption with lower levels of chaotrophs.
Use in agriculture
Natural freshwater diatomaceous earth is used in agriculture for grain storage as an anticaking agent, as well as an insecticide.[16] It is approved by the US Department of Agriculture as a feed supplement.
It is also used as a neutral anthelmintic (dewormer). Some farmers add it to their livestock and poultry feed to improve the health of animals.[17] "Food Grade Diatomaceous Earth" is widely available in agricultural feed supply stores. It is acceptable as organic feed additive for livestock.
Hydroponics
Freshwater diatomite can be used as a growing medium in hydroponic gardens.
It is also used as a growing medium in potted plants, particularly as bonsai soil. Bonsai enthusiasts use it as a soil additive, or pot a bonsai tree in 100% diatomaceous earth. Like perlite, vermiculite, and expanded clay, it retains water and nutrients, while draining fast and freely, allowing high oxygen circulation within the growing medium.
Marker in livestock nutrition experiments
Natural diatomaceous earth (dried, not calcined) is regularly used in livestock nutrition research as a source of acid insoluble ash (AIA), which is used as an indigestible marker. By measuring the content of AIA relative to nutrients in test diets and feces or digesta sampled from the terminal ileum (last third of the small intestine) the percentage of that nutrient digested can be calculated using the following equation:
- Where:
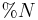 is percent Nutrient Digestibility
is percent Nutrient Digestibility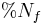 is the percent of nutrients in the feces
is the percent of nutrients in the feces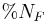 is the percent of nutrients in the feed
is the percent of nutrients in the feed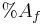 is the percent of AIA in the feces
is the percent of AIA in the feces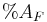 is the percent of AIA in the feed
is the percent of AIA in the feed
- And:
Natural diatomaceous earth (freshwater) is preferred by many researchers over chromic oxide, which has been widely used for the same purpose, but which is also a known carcinogen and therefore a potential hazard to research personnel.
Specific varieties
- Tripolite is the variety found in Tripoli, Libya.
- Bann clay is the variety found in the Lower Bann valley in Northern Ireland.
- Moler (Mo-clay) is the variety found in northwestern Denmark, especially on the islands of Fur and Mors.
- Fresh-water Food Grade Diatomaceous earth is the type used in US agriculture for grain storage, and as feed supplement.
Climatologic importance
The Earth's climate is affected by dust in the atmosphere, so locating major sources of atmospheric dust is important for climatology. Recent research indicates that surface deposits of diatomaceous earth play an important role. For instance, the largest single atmospheric dust source is the Bodélé depression in Chad, where storms push diatomite gravel over dunes, generating dust by abrasion.[18]
Safety considerations
The absorbent qualities of diatomite can result in a significant drying of the hands if handled without gloves. The flux-calcined form contains a highly crystalline form of silica, resulting in sharp edges. The sharpness of this version of the material makes it dangerous to breathe and a dust mask is recommended when working with it.
The type of hazard posed by inhalation depends on the form of the silica. Crystalline silica poses a serious inhalation hazard because it can cause silicosis. Amorphous silica can cause dusty lungs, but does not carry the same degree of risk as crystalline silica. Natural or dried diatomite generally contains very low percentages of crystalline silica. Diatomite produced for pool filters is treated with high heat (calcining) and a fluxing agent (soda ash), causing the formerly amorphous silicon dioxide to assume its crystalline form.
The crystalline silica content of the dust's particulate is regulated in the United States by the Occupational Safety and Health Administration (OSHA), and there are guidelines for the maximum amounts allowable in the product and in the air near the breathing zone of workers.[19]
The age and shape of diatoms
Each deposit of diatomaceous earth is different, with varying blends of pure diatomaceous earth combined with other natural clays and minerals.
The diatoms in each deposit contain different amounts of silica, depending on the age of the deposit. As well, the species of diatom may also differ among deposits.
The species of diatom is dependent upon the age and paleo-environment of the deposit. In turn, the shape of a diatom is determined by its species.
The shape of the diatoms contained in a deposit has not been proven to affect their functionality when it comes to the absorption of liquids, however certain applications, such as that for slugs and snails, do work best when a particular shaped diatom is used. For example, in the case of slugs and snails large, spiny diatoms work best to lacerate the outer shell of the insect.
Many deposits throughout British Columbia, Canada, such as Red Lake Earth, are from the Miocene age and contain a species of diatom known as Melosira granulate. These diatoms are approximately 12 to 13 million years old and are a small globular shape.
A deposit containing diatoms from this age can provide many more benefits than that of an older deposit. For example, diatoms from the Eocene age (approximately 40 to 50 millions year old) are not as effective in their ability to absorb fluids because older diatoms such as this recrystallize, their small pores becoming filled with silica.[20]
See also
References
- ^ a b c Antonides, Lloyd E. (1997) (PDF). Diatomite. U.S.G.S.. http://minerals.usgs.gov/minerals/pubs/commodity/diatomite/250497.pdf. Retrieved 2010-12-12.
- ^ http://home.cc.umanitoba.ca/~fieldspg/fields/de-test-p.pdf
- ^ http://www.humboldt-foundation.de/kosmos/kultur/2001_002.htm + Heinrich Küsel „Der Speicher“ v.1930
- ^ ELGA Berkefeld Water Treatment (Wasseraufbereitung)
- ^ http://www2.natpa.de/bonifatius/senken/p7.htm Über den früheren Abbau von Kieselgur im Vogelsberg/Hessen
- ^ http://homepages.compuserve.de/tmby100/kieselgur.htm Geschichte des Kieselgurabbaus in Klieken
- ^ a b Cummins, Arthur B., Diatomite, in Industrial Minerals and Rocks, 3rd ed. 1960, American Institute of Mining, Metallurgical, and Petroleum Engineers, pp. 303 - 319
- ^ Root, A.I. E.R. Root (March 1, 2005) The ABC and xyz of bee culture Kessinger Publishing p. 387 ISBN 978-1432626853 http://books.google.com/books?id=i0PoSYNEsh0C&pg=PA387&lpg=PA387&dq=diatomaceous+earth+%22does+not+remove%22&source=bl&ots=hs7-VtNc36&sig=EUbcCQhug9UXpJYMCLABzhUW2fc&hl=en&ei=w9DGTZjaN4nCsAPNxOmSAQ&sa=X&oi=book_result&ct=result&resnum=1&ved=0CBkQ6AEwAA#v=onepage&q=diatomaceous%20earth%20%22does%20not%20remove%22&f=false. Retrieved March 8, 2011
- ^ Staff. "Natural Methods for Controlling Fleas."http://www.xmission.com/~emailbox/fleas.htm
- ^ Lartigue, E. del C.; Rossanigo, C. E., 2004. Insecticide and anthelmintic assessment of diatomaceous earth in cattle. Veterinaria Argentina 21(209): 660-674
- ^ Fernandez MI, Woodward BW, Stromberg BE, 1998. Effect of diatomaceous earth as an anthelmintic treatment on internal parasites and feedlot performance of beef steers. Animal Science 66: 635-641
- ^ http://www.survival-center.com/foodfaq/ff17-oxy.htm Survival Information Center
- ^ http://www.dailyfinance.com/story/the-business-of-bedbugs/19627257/
- ^ Flynn, Thomas M. "Cryogenic Equipment and Cryogenic Systems Analysis." Cryogenic Engineering. Boca Raton [etc.: CRC, 2005. Print.
- ^ Goren R, Baykara T, Marsoglu M. A study on the purification of diatomite in hydrochloric acid (2002). Scand. J. of Metallurgy 31:115-119
- ^ Manitoba Agriculture, Food and Rural Initiatives
- ^ Diatomaceous Earth (DE)
- ^ Washington et al., Geophys. Res. Lett. 33 (2006) L09401 doi:10.1029/2006GL025827.
- ^ Inert Dusts at Kansas State University
- ^ "Diatoms". UCL London's Global University. http://www.ucl.ac.uk/GeolSci/micropal/diatom.html. Retrieved September 14, 2011.
External links
- International Chemical Safety Card 0248
- Diatomite: Statistics and Information - USGS
- Tripolite: Tripolite mineral data Citat: "...A diatomaceous earth consisting of opaline silica..."
- DIATOMACEOUS EARTH: A Non Toxic Pesticide