Dextroamphetamine
| Dextroamphetamine | |
|---|---|
|
(2S)-1-phenylpropan-2-amine[1] |
|
| Identifiers | |
| CAS number | 51-64-9 |
| PubChem | 5826 |
| ChemSpider | 5621 |
| UNII | TZ47U051FI |
| EC number | 200-112-1 |
| DrugBank | DB01576 |
| KEGG | D03740 |
| MeSH | Dextroamphetamine |
| ChEBI | CHEBI:4469 |
| ChEMBL | CHEMBL612 |
| ATC code | N06 |
| Beilstein Reference | 2205872 |
| Gmelin Reference | 1125854 |
| Jmol-3D images | Image 1 Image 2 |
|
|
|
|
| Properties | |
| Molecular formula | C9NH13 |
| Molar mass | 135.2062 g mol-1 |
| Exact mass | 135.104799421 g mol-1 |
| log P | 1.789 |
| Pharmacology | |
| Bioavailability | >75% (oral) |
| Routes of administration |
Insufflated, intravenous, oral, rectal, sublingual, vaporized |
| Metabolism | Hepatic |
| Elimination half-life |
10-28 h (average 12 h) |
| Excretion | ~45% (renal) |
| Legal status | Controlled (S8)(AU) |
| Pregnancy category |
B3(AU) C(US) |
| (verify) (what is: /?) Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Dextroamphetamine is a psychostimulant drug which is known to produce increased wakefulness and focus as well as decreased fatigue and decreased appetite.
Dextroamphetamine is the dextrorotatory, or "right-handed", stereoisomer of the amphetamine molecule. The amphetamine molecule has two stereoisomers; levoamphetamine and dextroamphetamine. Names for dextroamphetamine include d-amphetamine, dexamphetamine, dexamfetamine, and (S)-(+)-amphetamine, with brand names to include Dexedrine and Dextrostat.
The dextroamphetamine salts constitute around 75% of the ADHD drug Adderall[2]. Dextroamphetamine is also an active metabolite of the prodrug lisdexamfetamine (Vyvanse), as well as of several older N-substituted amphetamine prodrugs used as anorectics, such as clobenzorex (Asenlix), benzphetamine (Didrex), and amphetaminil (Aponeuron).
Contents |
Usage
Medical usage
Dextroamphetamine is used for the treatment of ADHD and narcolepsy.[3]
Dextroamphetamine may also be used for exogenous obesity and treatment-resistant depression.[4][5]
Military usage
The U.S. Air Force uses dextroamphetamine as one of its "go pills", given to pilots on long missions to help them remain focused and alert.[6][7][8][9] The Tarnak Farm incident was linked by media reports to the use of this drug on long term fatigued pilots. The military did not accept this explanation, citing the lack of similar incidents. Newer stimulant medications or awakeness promoting agents with different side effect profiles, such as modafinil are being investigated and sometimes issued for this reason.[6]
During the Vietnam War, Special Units of the US Military, such as MACV-SOG, were issued dextroamphetamine tablets. Due to the threat of misuse, these tablets were given to the Commanding Officer of the unit, and given out when needed.[10]
Experimental usage
Though such use remains out of the mainstream, dextroamphetamine has been successfully applied in the treatment of certain categories of depression as well as other psychiatric syndromes.[11] Such alternate uses include reduction of fatigue in cancer patients,[12] antidepressant treatment for HIV patients with depression and debilitating fatigue,[13] and early stage physiotherapy for severe stroke victims.[14] If physical therapy patients take dextroamphetamine while they practice their movements for rehabilitation, they may learn to move much faster than without dextroamphetamine, and in practice sessions with shorter lengths.[15]
Recreational usage
Along with methamphetamine, amphetamine and methylphenidate, dextroamphetamine is used as a recreational stimulant to induce euphoria and may be used as a study aid, social aid or party drug.
Contraindications
- Presence of seizures
- Advanced arteriosclerosis
- Hypersensitivity or idiosyncrasy to the sympathomimetic amines
- During or within 14 days following the administration of monoamine oxidase inhibitors (MAOIs) (hypertensive crisis may occur) [16]
Effects
Physical
Physical effects can include anorexia, hyperactivity, dilated pupils, flushing, restlessness, dry mouth, headache, tachycardia, bradycardia, tachypnea, hypertension, hypotension, hyperthermia, diaphoresis, diarrhea, constipation, blurred vision, dizziness, twitching, insomnia, numbness, palpitations, arrhythmias, tremors, dry and/or itchy skin, acne, pallor, and with chronic and/or high doses, convulsions, heart attack, stroke, and death can occur.[17][18][19][20][21]
Psychological
Psychological effects can include euphoria, anxiety, increased libido, alertness, concentration, increased energy, increased self-esteem, self-confidence, sociability, irritability, aggressiveness, psychosomatic disorders, psychomotor agitation, dermatillomania, delusions of grandiosity, hallucinations, excessive feelings of power and invincibility, repetitive and obsessive behaviors, paranoia, and with chronic and/or high doses, amphetamine psychosis can occur.[22][23]
Withdrawal
Withdrawal symptoms of dextroamphetamine primarily consist of fatigue, depression and an increased appetite. Symptoms may last for days with occasional use and weeks or months with chronic use, with severity dependent on the length of time and the amount of dextroamphetamine used. Withdrawal symptoms may also include anxiety, irritability, headaches, agitation, akathisia, hypersomnia (excessive sleeping), vivid or lucid dreams, deep REM sleep and suicidal ideation.[24][25][26]
Overdose
The Physician's 1991 Drug Handbook reports: "Symptoms of overdose include restlessness, tremor, hyperreflexia, tachypnea, confusion, aggressiveness, hallucinations, and panic." Dilated pupils are common with high doses. Repeated high doses may lead to manifestations of acute psychosis.
The fatal dose in humans is not precisely known, but in various species of rat generally ranges between 50 and 100 mg/kg, or a factor of 100 over what is required to produce noticeable psychological effects.[27][28] Although the symptoms seen in a fatal overdose are similar to those of methamphetamine, their mechanisms are not identical, as some substances which inhibit dextroamphetamine toxicity do not do so for methamphetamine.[29][30]
An extreme symptom of overdose is amphetamine psychosis, characterized by vivid visual, auditory, and sometimes tactile hallucinations. Many of its symptoms are identical to the psychosis-like state which follows long-term sleep deprivation, so it remains unclear whether these are solely the effects of the drug, or due to the long periods of sleep deprivation which are often undergone by the chronic user. Amphetamine psychosis, however, is extremely rare in individuals taking oral amphetamines at therapeutic doses; it is usually seen in cases of prolonged or high-dose intravenous (IV) for non-medicinal uses.[31]
Chemistry
Dextroamphetamine is the dextrorotatory stereoisomer of the amphetamine molecule, which can take two different forms. It is a slightly polar, weak base and is lipophilic.[32]
Formulations
Dextroamphetamine sulfate
In the United States, an instant release tablet preparation of the salt dextroamphetamine sulfate is available under the brand names Dexedrine and Dextrostat, in 5 mg and 10 mg strengths, and as a capsule preparation of controlled release dextroamphetamine sulfate, under the brand names Dexedrine SR and Dexedrine Spansule, in the strengths of 5 mg, 10 mg, and 15 mg. A bubblegum flavored oral solution is available under the brand name ProCentra, manufactured by FSC Pediatrics, which is designed to be an easier method of administration in children who have difficulty swallowing tablets, each 5 mL contains 5 mg dextroamphetamine.[33] In Australia dextroamphetamine is only available in bottles of 100 under the generic name Dexamphetamine as 5 mg instant release sulfate tablets.[34]
Lisdexamfetamine
Dextroamphetamine is the active metabolite of the prodrug lisdexamfetamine (L-lysine-d-amphetamine), available by the trademark name Vyvanse. Lisdexamfetamine is metabolised in the gastrointestinal tract, while dextroamphetamine's metabolism is hepatic.[35] Lisdexamfetamine is therefore an inactive compound until it is converted into an active compound by the digestive system. Although still rated as a Schedule II drug by the U.S. Drug Enforcement Administration, lisdexamfetamine has a slower onset and its route of administration is limited to being taken orally, unlike dextroamphetamine, Adderall, and methylphenidate, which can be insufflated to achieve a faster onset with a higher bioavailability. Vyvanse is marketed as once-a-day dosing as it provides a slow release of dextroamphetamine into the body. Vyvanse is available as capsules, and in six strengths; 20 mg, 30 mg, 40 mg, 50 mg, 60 mg, and 70 mg. The conversion rate of lisdexamfetamine to dextroamphetamine base is 0.2948[36] , so a 30 mg-strength Vyvanse capsule is molecularly equivalent to 8.844 mg dextroamphetamine base.
Mixed amphetamine salts
Another pharmaceutical that contains dextroamphetamine is Adderall. The drug formulation of Adderall (both controlled and instant release forms) is:
-
- One-quarter racemic (d,l-)amphetamine aspartate monohydrate
- One-quarter dextroamphetamine saccharin
- One-quarter dextroamphetamine sulfate
- One-quarter racemic (d,l-)amphetamine sulfate
Adderall is roughly three-quarters dextroamphetamine, with it accounting for 72.7% of the amphetamine base in Adderall (the remaining percentage is levoamphetamine).
An experiment with rats suggested Adderall’s inclusion of levoamphetamine provides the pharmaceutical with a quicker onset and longer clinical effect compared to pharmaceuticals exclusively formulated of dextroamphetamine. One study has shown that although the human brain usually has a preference for dextroamphetamine over levoamphetamine, certain children have a better clinical response to levoamphetamine.[37]
Amphetamine exists as two stereoisomers that differ in effects. The l- enantiomer (levoamphetamine) produces more cardiovascular and peripheral effects than the d- enantiomer (dextroamphetamine). At low doses, levoamphetamine produces greater arousal than dextroamphetamine, acting primarily on norepinephrine. At higher doses, dextroamphetamine has stimulant properties that are three to four times as potent as those of levoamphetamine, and acts primarily on dopamine, although few clinical studies of ADHD, have documented differences among d-, l- and racemic amphetamine.
Just as dextroamphetamine has greater central effects and fewer peripheral effects than levoamphetamine, methamphetamine, which is equipotent to dextroamphetamine in producing behavioral stimulant effects, has even fewer peripheral effects and greater central effects than dextroamphetamine.[38]
In relation to other over-the-counter ADD/ADHD pharmaceuticals, the d- isomer of racemic amphetamine is superior in bioavailibility and CNS stimulation.[39]
Pharmacology
Scientific findings have established that dextroamphetamine administration increases the activity of the phosphoinositol cycle via an indirect release of dopamine and noradrenaline. These results are the first time that this has been confirmed in humans.[40] Because dextroamphetamine is a substrate analog at monoamine transporters, at all doses, dextroamphetamine prevents the re-uptake of these neurotransmitters by competing with endogenous monoamines for uptake.[41] Transporter inhibition causes monoamines to remain in the synaptic cleft for a prolonged period (amphetamine inhibits monoamine reuptake in rats with a norepinephrine to dopamine ratio (NE:DA) of 1:1 and a norepinephrine to 5-hydroxytryptamine ratio (NE:5-HT) of about 100:1).[42]
At higher doses, when the concentration of dextroamphetamine is sufficient,[41] the drug can trigger direct release of norepinephrine and dopamine from the cytoplasmic transmitter pool, that is, dextroamphetamine will cause norepinephrine and dopamine efflux via transporter proteins, functionally reversing transporter action, which triggers a cascading release of catecholamines. This inversion leads to a release of large amounts of these neurotransmitters from the cytoplasm of the presynaptic neuron into the synapse, causing increased stimulation of post-synaptic receptors, inducing euphoria. Dextroamphetamine releases monoamines in rats with selectivity ratios of about NE:DA = 1:3.5 and NE:5-HT = 1:250, meaning that NE and DA are readily released, but release of 5-HT occurs at a 1/4 ration than of NE:DA.[43]
Dextroamphetamine increases dopamine release in the prefrontal cortex; activation of the dopamine-2 receptors inhibits glutamate release in the prefrontal cortex. Activation of the dopamine-1 receptors in the prefrontal cortex, however, results in elevated glutamate levels in the nucleus accumbens. An increase of the glutamate levels in the nucleus accumbens is the reason that dextroamphetamine has an ability to increase locomotor activity in rats. Serotonin also plays a role in dextroamphetamine's effect on glutamate levels; however, at therapeutic doses, dextroamphetamine has minuscule effect on the serotonin transporter (SERT).[44]
Pharmacokinetics
On average, about one half of a given dose is eliminated unchanged in the urine, while the other half is broken down into various metabolites (mostly benzoic acid).[45] However, the drug's half-life is highly variable because the rate of excretion is very sensitive to urinary pH. Under alkaline conditions, direct excretion is negligible and 95%+ of the dose is metabolized. Having an alkaline stomach will cause the drug to be absorbed faster through the stomach resulting in a higher blood level concentration of amphetamine. Having an alkaline bladder causes dextroamphetamine to be excreted more slowly from the blood and into the urine.
Alkalinization of the urine can decrease the renal elimination of amphetamines, both potentiating the strength and prolonging the mechanism of action, especially when ingested with sodium bicarbonate. There are also users who periodically misuse more than others, often using small amounts (1/2-1 teaspoon) of sodium bicarbonate mixed with water at 2-3 intervals following a single ingestion in order to achieve a longer euphoric effect. This method of administration may lead to a higher risk of intoxication, especially in children, in addition, tolerance and dependence develop rapidly.[46]
The main metabolic pathway is:
dextroamphetamine 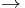 phenylacetone
phenylacetone 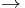 benzoic acid
benzoic acid 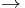 hippuric acid.
hippuric acid.
Another pathway, mediated by enzyme CYP2D6, is:
dextroamphetamine 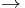 p-hydroxyamphetamine
p-hydroxyamphetamine 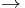 p-hydroxynorephedrine.
p-hydroxynorephedrine.
Although p-hydroxyamphetamine is a minor metabolite (~5% of the dose), it may have significant physiological effects as a norepinephrine analogue.[47]
Subjective effects are increased by larger doses, however, over the course of a given dose there is a noticeable divergence between such effects and drug concentration in the blood.[48] In particular, mental effects peak before maximal blood levels are reached, and decline as blood levels remain stable or even continue to increase.[49][50][51] This indicates a mechanism for development of acute tolerance, perhaps distinct from that seen in chronic use.[52] The long-term effects of amphetamines use on neural development in children has not been well established.[53] Based on a study in rats, amphetamine abuse during adolescence may impair adult working memory.[54]
History
Racemic amphetamine was first synthesized under the chemical name "phenylisopropylamine" in Berlin, 1887 by the Romanian chemist Lazar Edeleanu.[55] It was not widely marketed until 1932, when the pharmaceutical company Smith, Kline & French (now known as GlaxoSmithKline) introduced it in the form of the Benzedrine inhaler for use as a bronchodilator. Notably, the amphetamine contained in the Benzedrine inhaler was the liquid free-base,[n 1] not a chloride or sulfate salt.
Three years later, in 1935, the medical community became aware of the stimulant properties of amphetamine, specifically dextroamphetamine, and in 1937 Smith, Kline, and French introduced tablets under the tradename Dexedrine.[56] In the United States, Dexedrine was approved to treat narcolepsy, attention disorders, depression, and obesity. Dextroamphetamine was marketed in various other forms in the following decades, primarily by Smith, Kline, and French, such as several combination medications including a mixture of dextroamphetamine and amobarbital (a barbiturate) sold under the tradename Dexamyl and, in the 1950s, an extended release capsule (the "Spansule").[57]
It quickly became apparent that dextroamphetamine and other amphetamines had a high potential for misuse, although they were not heavily controlled until 1970, when the Comprehensive Drug Abuse Prevention and Control Act was passed by the United States Congress. Dextroamphetamine, along with other sympathomimetics, was eventually classified as Schedule II, the most restrictive category possible for a drug with a government sanctioned, recognized medical use.[58] Internationally, it has been available under the names AmfeDyn (Italy), Curban (US), Obetrol (Switzerland), Simpamina (Italy), Dexedrine (US & Canada), Dextropa (Portugal), and Stild (Spain).[59] According to UK pharmacies, the brand Dexedrine is no longer produced in the UK. It was apparently discontinued in 2010, most likely due to declining numbers being prescribed dextroamphetamine in the country.
In October 2010, GlaxoSmithKline sold the rights for Dexedrine Spansule to Amedra Pharmaceuticals (a subsidiary of CorePharma).[60]
References
- ^ "Dextroamphetamine - PubChem Public Chemical Database". The PubChem Prroject. USA: National Center for Biotechnology Information. 16 September 2004. Descriptors Computed from Structure. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=5826&loc=ec_rcs. Retrieved 26 September 2011.
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/011522s040lbl.pdf | Adderall Drug Insert (revised: March 2007)
- ^ U.S. Food and Drug Administration | GlaxoSmithKline Prescribing Information
- ^ Answers.com | Amphetamine; Dextroamphetamine extended-release capsules
- ^ Dexedrine Addiction | The U.S. Military Needs Its Dexedrine
- ^ a b Air Force scientists battle aviator fatigue
- ^ U.S. Pilots Stay Up Taking 'Uppers'
- ^ Emonson, DL; Vanderbeek, RD (1995). "The use of amphetamines in U.S. Air Force tactical operations during Desert Shield and Storm.". Aviation, space, and environmental medicine 66 (3): 260–3. PMID 7661838.
- ^ ‘Go pills’: A war on drugs?, msnbc, Jan. 9, 2003
- ^ pharmacymap.info | Dextroamphetamine
- ^ Warneke L (1990). "Psychostimulants in psychiatry". Can J Psychiatry 35 (1): 3–10. PMID 2180548.
- ^ Breitbart W, Alici Y (2010). "Psychostimulants for cancer-related fatigue.". J Natl Compr Canc Netw 8 (8): 933–42. PMID 20870637.
- ^ Wagner G, Rabkin R (2000). "Effects of dextroamphetamine on depression and fatigue in men with HIV: a double-blind, placebo-controlled trial". J Clin Psychiatry 61 (6): 436–40. doi:10.4088/JCP.v61n0608. PMID 10901342.
- ^ Martinsson L, Yang X, Beck O, Wahlgren N, Eksborg S (2003). "Pharmacokinetics of dexamphetamine in acute stroke". Clin Neuropharmacol 26 (5): 270–6. doi:10.1097/00002826-200309000-00012. PMID 14520168.
- ^ Butefisch CM et al. (2002). "Modulation of Use-Dependent Plasticity by D-Amphetamine". Annals of Neurology 51 (1): 59–68. doi:10.1002/ana.10056. PMID 11782985.
- ^ RxList | Dexedrine (Dextroamphetamine)
- ^ Erowid Amphetamines Vault | Effects
- ^ Amphetamine; Facts | Alcoholism and Drug Addiction Research Foundation, Toronto Canada
- ^ Amphetamines | Better Health Channel
- ^ Dextroamphetamine (Oral Route) | MayoClinic.com
- ^ Vitiello B (2008). "Understanding the Risk of Using Medications for ADHD with Respect to Physical Growth and Cardiovascular Function". Child Adolesc Psychiatr Clin N Am 17 (2): 459–74, xi. doi:10.1016/j.chc.2007.11.010. PMC 2408826. PMID 18295156. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2408826.
- ^ Erowid Amphetamines Vault: Effects
- ^ Amphetamines | Merck Sharp & Dohme Corp.
- ^ Symptoms of Amphetamine withdrawal | WrongDiagnosis.com
- ^ eMedTV | Dextroamphetamine Withdrawals
- ^ Drug Abuse Help | Dexedrine Information
- ^ Miczek K (1979). "A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine" (Abstract). Psychopharmacology (Berl) 60 (3): 253–9. doi:10.1007/BF00426664. PMID 108702. http://www.springerlink.com/content/v11jj22163t30326/.
- ^ Grilly D, Loveland A (2001). "What is a "low dose" of d-amphetamine for inducing behavioral effects in laboratory rats?". Psychopharmacology (Berl) 153 (2): 155–69. doi:10.1007/s002130000580. PMID 11205415.
- ^ Derlet R, Albertson T, Rice P (1990). "Antagonism of cocaine, amphetamine, and methamphetamine toxicity". Pharmacol Biochem Behav 36 (4): 745–9. doi:10.1016/0091-3057(90)90071-O. PMID 2217500.
- ^ Derlet R, Albertson T, Rice P (1990). "The effect of SCH 23390 against toxic doses of cocaine, d-amphetamine and methamphetamine". Life Sci 47 (9): 821–7. doi:10.1016/0024-3205(90)90555-6. PMID 2215083.
- ^ LS Goodman, A Gilman (1970). The Pharmacological Basis of Therapeutics (7th ed.). New York: Macmillan Co..
- ^ DrugBank | Showing drug card for Dextroamphetamine (DB01576)
- ^ FSC Laboratories: ProCentra (dextroamphetamine sulfate | 5 mg/5 mL Oral Solution)
- ^ Australian Prescriber | Stimulant treatment for attention deficit hyperactivity disorder
- ^ FDA Approval of Vyvanse Pharmacological Reviews Pages 18 and 19
- ^ Moore, Elaine.The Amphetamine Debate: The Use of Adderall, Ritalin and Related Drugs for Behavior Modification, Neuroenhancement and Anti-Aging Purposes. McFarland, 2010, p. 91.
- ^ Arnold (2000). "Methylphenidate vs Amphetamine: Comparative Review". Journal of Attention Disorders 3 (4): 200–211. doi:10.1177/108705470000300403.
- ^ NCBI | Potential Adverse Effects of Amphetamine Treatment on Brain and Behavior: A Review
- ^ Glaser, et al.; Thomas, Theresa C.; Joyce, B. Matthew; Castellanos, F. Xavier; Gerhardt, Greg A. (2005). "Differential Effects of Amphetamine Isomers on Dopamine in the Rat Striatum and Nucleus Accumbens Core". Psychopharmacology 178 (2–3): 250–258 (Pages: 255,256). doi:10.1007/s00213-004-2012-6. PMID 15719230.
- ^ InterScience | Dextroamphetamine increases phosphoinositol cycle activity in volunteers: an MRS study
- ^ a b Kuczenski R et al. (1 February 1995). "Hippocampus Norepinephrine, Caudate Dopamine and Serotonin, and Behavioral Responses to the Stereoisomers of Amphetamine and Methamphetamine". The Journal of Neuroscience 15 (2): 1308–1317. PMID 7869099. http://www.jneurosci.org/cgi/reprint/15/2/1308.
- ^ Rothman, et al. "Amphetamine-Type Central Nervous System Stimulants Release Norepinephrine more Potently than they Release Dopamine and Serotonin." (2001): Synapse 39, 32–41 (Table V. on page 37)
- ^ Patrick, and Markowitz; Markowitz, John S. (1997). "Pharmacology of Methylphenidate, Amphetamine Enantiomers and Pemoline in Attention-Deficit Hyperactivty Disorder". Human Psychopharmacology 12 (6): 527–546 (Page:530). doi:10.1002/(SICI)1099-1077(199711/12)12:6<527::AID-HUP932>3.0.CO;2-U.
- ^ Shoblock J, Sullivan E, Maisonneuve I, Glick S (2003). "Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats". Psychopharmacology (Berl) 165 (4): 359–69. doi:10.1007/s00213-002-1288-7. PMID 12491026.
- ^ Mofenson H, Greensher J (1975). "Letter: Physostigmine as an antidote: use with caution". J Pediatr 87 (6 Pt 1): 1011–2. doi:10.1016/S0022-3476(75)80946-2. PMID 1185381.
- ^ Drug Interactions Checker Results | sodium bicarbonate + dextroamphetamine | Drugs.com
- ^ Rangno R, Kaufmann J, Cavanaugh J, Island D, Watson J, Oates J (1973). "Effects of a False Neurotransmitter, p-Hydroxynorephedrine, on the Function of Adrenergic Neurons in Hypertensive Patients" (Scanned copy). J Clin Invest 52 (4): 952–60. doi:10.1172/JCI107260. PMC 302343. PMID 4348345. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=302343.
- ^ Asghar S, Tanay V, Baker G, Greenshaw A, Silverstone P (2003). "Relationship of plasma amphetamine levels to physiological, subjective, cognitive and biochemical measures in healthy volunteers". Hum Psychopharmacol 18 (4): 291–9. doi:10.1002/hup.480. PMID 12766934.
- ^ Angrist B, Corwin J, Bartlik B, Cooper T (1987). "Early pharmacokinetics and clinical effects of oral D-amphetamine in normal subjects". Biol Psychiatry 22 (11): 1357–68. doi:10.1016/0006-3223(87)90070-9. PMID 3663788.
- ^ Brown G, Hunt R, Ebert M, Bunney W, Kopin I (1979). "Plasma levels of d-amphetamine in hyperactive children. Serial behavior and motor responses" (Abstract). Psychopharmacology (Berl) 62 (2): 133–40. doi:10.1007/BF00427126. PMID 111276. http://www.springerlink.com/content/w14m559771164631/.
- ^ Brauer L, Ambre J, De Wit H (1996). "Acute tolerance to subjective but not cardiovascular effects of d-amphetamine in normal, healthy men". J Clin Psychopharmacol 16 (1): 72–6. doi:10.1097/00004714-199602000-00012. PMID 8834422.
- ^ MacKenzie R, Heischober B (1997). "Methamphetamine". Pediatr Rev 18 (9): 305–9. doi:10.1542/pir.18-9-305. PMID 9286149.
- ^ RxList | Adderall
- ^ "Amphetamine use in adolescence may impair adult working memory". http://news.illinois.edu/news/09/1021amphetamine.html. Retrieved 2009-10-22.
- ^ Help For Dexedrine Addicts | Dexedrine Rehab Centers For Addicts
- ^ Dexedrine | Drug information from Medic8.com
- ^ Information on Dexedrine: A Quick Review | Weitz & Luxenberg
- ^ Prescription Forgery | Handwriting Services International
- ^ Pharmaceutical Manufacturing Encyclopedia (2nd edition), Marshall Sittig, Volume 1, Noyes Publications ISBN 978-0-8155-1144-1
- ^ "Dexedrine FAQs". http://www.dexedrine.net/faq.asp.
Notes
- ^ Free-base form amphetamine is a volatile oil, hence the efficacy of the inhalers.
Further reading
- Dexamphetamine 1845887055 at GPnotebook
- Package inserts: "United States". http://www.dexedrine.com/docs/dexedrine_PI.pdf."New Zealand". http://www.medsafe.govt.nz/profs/Datasheet/d/Dexamphetaminesulphatetab.pdf. "Canada". http://www.mentalhealth.com/drug/p30-d04.html.
- Poison Information Monograph (PIM 178: Dexamphetamine Sulphate)
External links
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||