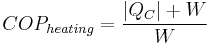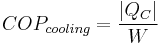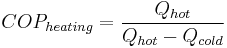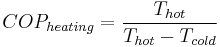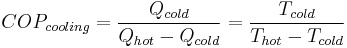Coefficient of performance
The coefficient of performance or COP (sometimes CP), of a heat pump is the ratio of the change in heat at the "output" (the heat reservoir of interest) to the supplied work.
Contents |
Equation
The equation is:
where
The COP for heating and cooling are thus different, because the heat reservoir of interest is different. When one is interested in how well a machine cools, the COP is the ratio of the heat removed from the cold reservoir to input work. However, for heating, the COP is the ratio to input work of the heat removed from the cold reservoir plus the heat added to the hot reservoir by the input work:
where
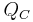 is the heat removed from the cold reservoir.
is the heat removed from the cold reservoir.
Derivation
According to the first law of thermodynamics, in a reversible system we can show that 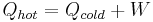 and
and 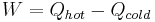 , where
, where 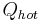 is the heat given off by the hot heat reservoir and
is the heat given off by the hot heat reservoir and 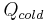 is the heat taken in by the cold heat reservoir.
is the heat taken in by the cold heat reservoir.
Therefore, by substituting for W,
For a heat pump operating at maximum theoretical efficiency (i.e. Carnot efficiency), it can be shown that 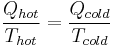 and
and 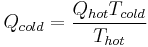 , where
, where 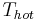 and
and 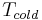 are the absolute temperatures of the hot and cold heat reservoirs respectively.
are the absolute temperatures of the hot and cold heat reservoirs respectively.
At maximum theoretical efficiency,
Which is equal to the inverse of the ideal Carnot cycle efficiency because a heat pump is a heat engine operating in reverse. Similarly,
It can also be shown that 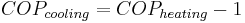 . Note that these equations must use the absolute temperature (the Kelvin or Rankine scale.)
. Note that these equations must use the absolute temperature (the Kelvin or Rankine scale.)
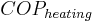 applies to heat pumps and
applies to heat pumps and 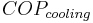 applies to air conditioners or refrigerators. For heat engines, see Efficiency. Values for actual systems will always be less than these theoretical maximums. In Europe, ground source heat pump units are standard tested at
applies to air conditioners or refrigerators. For heat engines, see Efficiency. Values for actual systems will always be less than these theoretical maximums. In Europe, ground source heat pump units are standard tested at 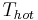 is 35 °C (95 °F) and
is 35 °C (95 °F) and 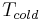 is 0 °C (32 °F). According to the above formula, the maximum achievable COP would be 8.8. Test results of the best systems are around 4.5. When measuring installed units over a whole season and one also counts the energy needed to pump water through the piping systems, then seasonal COP's are around 3.5 or less. This indicates room for improvement.
is 0 °C (32 °F). According to the above formula, the maximum achievable COP would be 8.8. Test results of the best systems are around 4.5. When measuring installed units over a whole season and one also counts the energy needed to pump water through the piping systems, then seasonal COP's are around 3.5 or less. This indicates room for improvement.
Improving COP
As the formula shows, to improve the COP of a heat pump system, one needs to reduce the temperature gap 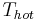 minus
minus 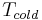 at which the system works. For a heating system this would mean two things. One is to reduce output temperature to around 30 °C (86 °F) which requires piped floor- or wall- or ceiling heating, or oversized water to air heaters. The other is to increase input temperature (by using an oversized ground source). For an air cooler, COP could be improved by using ground water as an input instead of air, and by reducing temperature drop on output side through increasing air flow. For both systems, also increasing the size of pipes and air canals would help to reduce noise and the energy consumption of pumps (and ventilators).
at which the system works. For a heating system this would mean two things. One is to reduce output temperature to around 30 °C (86 °F) which requires piped floor- or wall- or ceiling heating, or oversized water to air heaters. The other is to increase input temperature (by using an oversized ground source). For an air cooler, COP could be improved by using ground water as an input instead of air, and by reducing temperature drop on output side through increasing air flow. For both systems, also increasing the size of pipes and air canals would help to reduce noise and the energy consumption of pumps (and ventilators).
Also the heat pump itself can be improved a lot. The two most simple ways to improve heat pump units, is to double the size of the internal heat exchangers relative to the power of the compressor, and to reduce the system's internal temperature gap over the compressor. This last measure however, makes such heat pumps unsuitable to produce output above roughly 40 °C (104 °F) which means that a separate machine is needed for producing hot tap water.
Example
A geothermal heat pump operating at 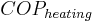 3.5 provides 3.5 units of heat for each unit of energy consumed (i.e. 1 kWh consumed would provide 3.5 kWh of output heat). The output heat comes from both the heat source and 1 kWh of input energy, so the heat-source is cooled by 2.5 kWh, not 3.5 kWh.
3.5 provides 3.5 units of heat for each unit of energy consumed (i.e. 1 kWh consumed would provide 3.5 kWh of output heat). The output heat comes from both the heat source and 1 kWh of input energy, so the heat-source is cooled by 2.5 kWh, not 3.5 kWh.
A heat pump of 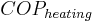 3.5, such as in the example above, could be less expensive to use than even the most efficient gas furnace except in areas where the electricity cost per unit is higher than 3.5 times the cost of natural gas (i.e. Connecticut or New York City).
3.5, such as in the example above, could be less expensive to use than even the most efficient gas furnace except in areas where the electricity cost per unit is higher than 3.5 times the cost of natural gas (i.e. Connecticut or New York City).
A heat pump cooler operating at 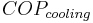 2.0 removes 2 units of heat for each unit of energy consumed (e.g. an air conditioner consuming 1 kWh would remove 2 kWh of heat from a building's air).
2.0 removes 2 units of heat for each unit of energy consumed (e.g. an air conditioner consuming 1 kWh would remove 2 kWh of heat from a building's air).
Given the same energy source and operating conditions, a higher COP heat pump will consume less purchased energy than one with a lower COP. The overall environmental impact of a heating or air conditioning installation depends on the source of energy used as well as the COP of the equipment. The operating cost to the consumer depenends on the cost of energy as well as the COP or efficiency of the unit. Some areas provide two or more sources of energy, for example, natural gas and electricity. A high COP of a heat pump may not entirely overcome a relatively high cost for electicity compared with the same heating value from natural gas.
For example, the 2009 US average price per therm (100,000 BTU) of electricity was $3.38 while the average price per therm of natural gas was $1.16.[1] Using these prices, a heat pump with a COP of 3.5 in moderate climate would cost $0.97[2] to provide one therm of heat, while a high efficiency gas furnace with 95% efficiency would cost $1.22[3] to provide one therm of heat. With these average prices, the heat pump costs 20% less[4] to provide the same amount of heat. At 0 °F (-18 °C) COP is much lower. Then, the same system costs as much to operate as an efficient gas heater. The yearly savings will depend on the actual cost of electricity and natural gas, which can both vary widely.
However, a COP may help make a determination of system choice based on carbon contribution. Although a heat pump may cost more to operate than a conventional natural gas or electric heater, depending on the source of electricity generation in one's area, it may contribute less net carbon dioxide to the environment than burning natural gas or heating fuel. If locally no green electricity is available, then carbon wise the best option would be to drive a heat pump on piped gas or oil, to store excess heat in the ground source for use in winter, while using the same machine also for producing electricity with a built-in Stirling engine.
Conditions of use
While the COP is partly a measure of the efficiency of a heat pump, it is also a measure of the conditions under which it is operating: the COP of a given heat pump will rise as the input temperature increases or the output temperature decreases because it is linked to a warm temperature distribution system like underfloor heating.
See also
- Seasonal energy efficiency ratio (SEER)
- Heating seasonal performance factor (HSPF)
- Thermal efficiency
- Vapor-compression refrigeration
- Air conditioner
- HVAC
- Heat Pump
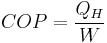
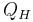 is the
is the 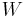 is the
is the