Chemotaxis
Chemotaxis is the phenomenon in which somatic cells, bacteria, and other single-cell or multicellular organisms direct their movements according to certain chemicals in their environment. This is important for bacteria to find food (for example, glucose) by swimming towards the highest concentration of food molecules, or to flee from poisons (for example, phenol). In multicellular organisms, chemotaxis is critical to early development (e.g. movement of sperm towards the egg during fertilization) and subsequent phases of development (e.g. migration of neurons or lymphocytes) as well as in normal function. In addition, it has been recognized that mechanisms that allow chemotaxis in animals can be subverted during cancer metastasis.
Positive chemotaxis occurs if the movement is toward a higher concentration of the chemical in question. Conversely, negative chemotaxis occurs if the movement is in the opposite direction.
Contents |
History of chemotaxis research
Neutrophils are the body's first line of defense against bacterial infections. After leaving nearby blood vessels, these cells recognize chemicals produced by bacteria in a cut or scratch and migrate "toward the smell". The above neutrophils were placed in a gradient of fMLP (N-formyl-methionine-leucine-phenylalanine), a peptide chain produced by some bacteria. Although migration of cells was detected from the early days of the development of microscopy (Leeuwenhoek), erudite description of chemotaxis was first made by T W. Engelmann (1881) and W.F. Pfeffer (1884) in bacteria and H.S. Jennings (1906) in ciliates. The Nobel Prize laureate I. Metchnikoff also contributed to the study of the field with investigations of the process as an initial step of phagocytosis. The significance of chemotaxis in biology and clinical pathology was widely accepted in the 1930s. The most fundamental definitions belonging to the phenomenon were also drafted by this time. The most important aspects in quality control of chemotaxis assays were described by H. Harris in the 1950s. In the 1960s and 1970s, the revolution of modern cell biology and biochemistry provided a series of novel techniques which became available to investigate the migratory responder cells and subcellular fractions responsible for chemotactic activity. The pioneering works of J. Adler represented a significant turning point in understanding the whole process of intracellular signal transduction of bacteria.[1]
On November 3, 2006, Dr. Dennis Bray of University of Cambridge was awarded the Microsoft Award for his work on chemotaxis on E. coli.[2][3]
Chemoattractants and chemorepellents
Chemoattractants and chemorepellents are inorganic or organic substances possessing chemotaxis-inducer effect in motile cells. Effects of chemoattractants are elicited via described or hypothetic chemotaxis receptors, the chemoattractant moiety of a ligand is target cell specific and concentration dependent. Most frequently investigated chemoattractants are formyl peptides and chemokines. Responses to chemorepellents result in axial swimming and they are considered a basic motile phenomena in bacteria. The most frequently investigated chemorepellents are inorganic salts, amino acids and some chemokines.
Bacterial chemotaxis
Some bacteria, such as E. coli, have several flagella per cell (4–10 typically). These can rotate in two ways :
- Counter-clockwise rotation aligns the flagella into a single rotating bundle, causing the bacterium to swim in a straight line.
- Clockwise rotation breaks the flagella bundle apart such that each flagellum points in a different direction, causing the bacterium to tumble in place.
The directions of rotation are given for an observer outside the cell looking down the flagella toward the cell.
Behavior
The overall movement of a bacterium is the result of alternating tumble and swim phases. If one watches a bacterium swimming in a uniform environment, its movement will look like a random walk with relatively straight swims interrupted by random tumbles that reorient the bacterium. Bacteria such as E. coli are unable to choose the direction in which they swim, and are unable to swim in a straight line for more than a few seconds due to rotational diffusion. In other words, bacteria "forget" the direction in which they are going. By repeatedly evaluating their course, and adjusting if they are moving in the wrong direction, bacteria can direct their motion to find favorable locations with high concentrations of attractants (usually food) and avoid repellents (usually poisons).
In the presence of a chemical gradient bacteria will chemotax, or direct their overall motion based on the gradient. If the bacterium senses that it is moving in the correct direction (toward attractant/away from repellent), it will keep swimming in a straight line for a longer time before tumbling. If it is moving in the wrong direction, it will tumble sooner and try a new direction at random. In other words, bacteria like E. coli use temporal sensing to decide whether life is getting better or worse. In this way, it finds the location with the highest concentration of attractant (usually the source) quite well. Even under very high concentrations, it can still distinguish very small differences in concentration. Fleeing from a repellent works with the same efficiency.
This purposeful random walk is a result of simply choosing between two methods of random movement; namely tumbling and straight swimming. In fact, chemotactic responses such as forgetting direction and choosing movements resemble the decision-making abilities of higher life-forms with brains that process sensory data.
The helical nature of the individual flagellar filament is critical for this movement to occur. As such, the protein that makes up the flagellar filament, flagellin, is quite similar among all flagellated bacteria. Vertebrates seem to have taken advantage of this fact by possessing an immune receptor (TLR5) designed to recognize this conserved protein.
As in many instances in biology, there are bacteria that do not follow this rule. Many bacteria, such as Vibrio, are monoflagellated and have a single flagellum at one pole of the cell. Their method of chemotaxis is different. Others possess a single flagellum that is kept inside the cell wall. These bacteria move by spinning the whole cell, which is shaped like a corkscrew.[4]
Signal transduction
Chemical gradients are sensed through multiple transmembrane receptors, called methyl-accepting chemotaxis proteins (MCPs), which vary in the molecules that they detect. These receptors may bind attractants or repellents directly or indirectly through interaction with proteins of periplasmatic space. The signals from these receptors are transmitted across the plasma membrane into the cytosol, where Che proteins are activated. The Che proteins alter the tumbling frequency, and alter the receptors.
Flagellum regulation
The proteins CheW and CheA bind to the receptor. The activation of the receptor by an external stimulus causes autophosphorylation in the histidine kinase, CheA, at a single highly conserved histidine residue. CheA in turn transfers phosphoryl groups to conserved aspartate residues in the response regulators CheB and CheY [ note: CheA is a histidine kinase and it does not actively transfer the phosphoryl group. The response regulator CheB takes the phosphoryl group from CheA]. This mechanism of signal transduction is called a two-component system and is a common form of signal transduction in bacteria. CheY induces tumbling by interacting with the flagellar switch protein FliM, inducing a change from counter-clockwise to clockwise rotation of the flagellum. Change in the rotation state of a single flagellum can disrupt the entire flagella bundle and cause a tumble.
Receptor regulation
CheB, when activated by CheA, acts as a methylesterase, removing methyl groups from glutamate residues on the cytosolic side of the receptor. It works antagonistically with CheR, a methyltransferase, which adds methyl residues to the same glutamate residues. If the level of an attractant remains high, the level of phosphorylation of CheA (and therefore CheY and CheB) will remain low, the cell will swim smoothly, and the level of methylation of the MCPs will increase (because CheB-P is not present to demethylate). However, the MCPs no longer respond to the attractant when they are fully methylated. Therefore, even though the level of attractant might remain high, the level of CheA-P (and CheB-P) increases and the cell begins to tumble. However, now the MCPs can be demethylated by CheB-P, and when this happens, the receptors can once again respond to attractants. The situation is the opposite with regard to repellents (fully methylated MCPs respond best to repellents, while least methylated MCPs respond worst to repellents). This regulation allows the bacterium to 'remember' chemical concentrations from the recent past, a few seconds, and compare them to those it is currently experiencing, thus 'know' whether it is traveling up or down a gradient. Although the methylation system accounts for the wide range of sensitivity [5] that bacteria have to chemical gradients, other mechanisms are involved in increasing the absolute value of the sensitivity on a given background. Well established examples are the ultra-sensitive response of the motor to the CheY-P signal, and the clustering of chemoreceptors.[6][7]
Eukaryotic chemotaxis
The mechanism by which eukaryotic cells chemotax is quite different from that in bacteria; however, sensing of chemical gradients is still a crucial step in the process. Due to their size, prokaryotes cannot detect effective concentration gradients, therefore these cells scan and evaluate their environment by a constant swimming (consecutive steps of straight swims and tumbles). In contrast to prokaryotes, the size of eukaryotic cells allows for the possibility of detecting gradients, which results in a dynamic and polarized distribution of receptors. Induction of these receptors by chemoattractants or chemorepellents results in migration towards or away from the chemotactic substance.
Levels of receptors, intracellular signalling pathways and the effector mechanisms all represent diverse, eukaryotic type components. In eukaryotic unicellular cells, ameboid movement and cilium or the eukaryotic flagellum are the main effectors (e.g. Amoeba or Tetrahymena).[8][9] Some eukaryotic cells of higher vertebrate origin, such as immune cells also move to where they need to be. Besides immune competent cells (granulocyte, monocyte, lymphocyte) a large group of cells - considered previously to be fixed into tissues - are also motile in special physiological (e.g. mast cell, fibroblast, endothelial cells)or pathological conditions (e.g. metastases). Chemotaxis has high significance in the early phases of embryogenesis as development of germ layers is guided by gradients of signal molecules.
Motility
Unlike motility in bacterial chemotaxis, the mechanism by which eukaryotic cells physically move is unclear. There appear to be mechanisms by which an external chemotactic gradient is sensed and turned into an intracellular PIP3 gradient, which results in a gradient and the activation of a signaling pathway, culminating in the polymerisation of actin filaments. The growing distal end of actin filaments develops connections with the internal surface of the plasma membrane via different sets of peptides and results in the formation of pseudopods. Cilia of eukaryotic cells can also produce chemotaxis; in this case it is mainly a Ca2+ dependent induction of the microtubular system of the basal body and the beat of the 9+2 microtubules within cilia. The orchestrated beating of hundreds of cilia is synchronized by a submembranous system built between basal bodies. The details of the signaling pathways are still not totally clear.
Although chemotaxis is the most frequently studied form of migration there are several other forms of locomotion in the cellular level.
- Chemokinesis is also induced by molecules of the liquid phase of the surrounding environment; however, the response elicited is a not vectorial, random taxis. Neither amplitude nor frequency of motion has characteristic, directional components as this behaviour provides more scanning of the environment than migration between two distinct points.
- In haptotaxis the gradient of the chemoattractant is expressed or bound on a surface, in contrast to the classical model of chemotaxis, in which the gradient develops in a soluble fluid. The most common biologically active haptotactic surface is the extracellular matrix (ECM); the presence of bound ligands is responsible for induction of transendothelial migration and angiogenesis.
- Necrotaxis embodies a special type of chemotaxis when the chemoattractant molecules are released from necrotic or apoptotic cells. Depending on the chemical character of released substances, necrotaxis can accumulate or repel cells, which underlines the pathophysiological significance of this phenomenon.
Receptors
For the most part, eukaryotic cells sense the presence of chemotactic stimuli through the use of 7-transmembrane (or serpentine) heterotrimeric G-protein coupled receptors. This class of receptors is huge, representing a significant portion of the genome. Some members of this gene superfamily are used in eyesight (rhodopsins) as well as in olfaction (smelling). The main classes of professional chemotaxis receptors are triggered by formyl peptides - formyl peptide receptors (FPR), chemokines - chemokine receptors (CCR or CXCR) and leukotrienes - leukotriene receptors (BLT); however, induction of a wide set of membrane receptors (e.g. amino acids, insulin, vasoactive peptides) also elicit migration of the cell.
Chemotactic selection
While some chemotaxis receptors are expressed in the surface membrane with long-term characteristics as they are determined genetically, others have short-term dynamics as they are assembled ad hoc in the presence of the ligand. The diverse features of the chemotaxis receptors and ligands allows for the possibility of selecting chemotactic responder cells with a simple chemotaxis assay. By chemotactic selection we can determine whether a still uncharacterized molecule acts via the long- or the short-term receptor pathway. The term chemotactic selection is also used to designate a technique which separates eukaryotic or prokaryotic cells according to their chemotactic responsiveness to selector ligands.[10]
Chemotactic ligands
The number of molecules capable of eliciting chemotactic responses is relatively high, and we can distinguish primary and secondary chemotactic molecules. The main groups of the primary ligands are as follows:
- Formyl peptides are di-, tri-, tetrapeptides of bacterial origin (see formyl group on the N terminus of the peptide). They are released from bacteria in vivo or after decomposition of the cell. A typical member of this group is the N-formylmethionyl-leucyl-phenylalanine (fMLF or fMLP in references). The bacterial origin fMLF as a key component of inflammation has characteristic chemoattractant effects in neutrophil granulocytes and monocytes.
- Complement 3a (C3a) and complement 5a (C5a) are intermediate products of complement cascade. Their synthesis is joined to the three alternative pathways (classical, lectin dependent and alternative) of complement activation by a convertase enzyme. The main target cells of these derivaties are neutrophil granulocytes and monocytes as well.
- Chemokines belong to a special class of cytokines. Their groups (C, CC, CXC, CX3C chemokines) represent not only structurally related molecules with a special arrangement of disulfide bridges, but their target cell specificity is also diverse: CC chemokines act on monocytes (e.g. RANTES), CXC chemokines are neutrophil granulocyte specific (e.g. IL-8).
Investigations of the three-dimensional structures of chemokines proved that a characteristic composition of beta-sheets and an alpha helix provides expression of sequences required for interaction with the chemokine receptors. Formation of dimers and their increased biological acitvity was demonstrated by crystallography of several chemokines e.g. IL-8.
- Leukotrienes belong to the group eicosanoids. They are significant lipid mediators of the arachidonic acid cascade converted by 5-lipoxigenase. Their predominant member is leukotriene B4 (LTB4) which elicits adhesion, chemotaxis and aggregation of leukocytes. The characteristic chemoattractant effect of LTB4 is induced via G-protein linked seven-transmembrane spanning leukotriene receptors which are highly expressed in inflammation and allergy.
Chemotactic range fitting (CRF)
Chemotactic responses elicited by the ligand-receptor interactions are distinguished generally upon the optimal effective concentration(s) of the ligand. Nevertheless, correlation of the amplitude elicited and ratio of the responder cells compared to the total number are also characteristic features of the chemotactic signaling. Investigations of ligand families (e.g. amino acids or oligo peptides) proved that there is a fitting of ranges (amplitudes; number of responder cells) and chemotactic activities: chemoattractant moiety is accompanied by wide ranges, while chemorepellent character by narrow ranges.
Clinical significance
A changed migratory potential of cells has relatively high importance in the development of several clinical symptoms and syndromes. Altered chemotactic activity of extracellular (e.g. Escherichia coli) or intracellular (e.g. Listeria monocytogenes) pathogens itself represents a significant clinical target. Modification of endogenous chemotactic ability of these microorganisms by pharmaceutical agents can decrease or inhibit the ratio of infections or spreading of infectious diseases. Apart from infections, there are some other diseases where impaired chemotaxis is the primary etiological factor, as in Chediak-Higashi syndrome where giant intracellular vesicles inhibit normal migration of cells.
| Type of disease | Chtx. increased | Chtx. decreased |
|---|---|---|
| Infections | inflammations | AIDS, Brucellosis |
| Chtx. results the disease | - | Chediak-Higashi syndrome, Kartagener syndrome |
| Chtx. is affected | atherosclerosis, arthritis, periodontitis, psoriasis, reperfusion injury, metastatic tumors | multiple sclerosis, Hodgkin disease, male infertility |
| Intoxications | asbestos, benzpyrene | salts of Hg and Cr, ozone (O3) |
Mathematical models
Several mathematical models of chemotaxis were developed depending on the type of
- migration (e.g. basic differences of bacterial swimming, movement of unicellular eukaryotes with cilia/flagellum and ameboid migration);
- physico-chemical characteristics of the chemicals (e.g. diffusion) working as ligands;
- biological characteristics of the ligands (attractant, neutral and repellent molecules;
- assay systems applied to evaluate chemotaxis (see incubation times, development and stability of concentration gradients);
- other environmental effects possessing direct or indirect influence on the migration (lighting, temperature, magnetic fields etc.)
Although interactions of the factors listed above make the behavior of the solutions of mathematical models of chemotaxis rather complex, it is possible to describe the basic phenomenon of chemotaxis-driven motion in a straightforward way. Indeed, let us denote with 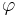 the spatially non-uniform concentration of the chemo-attractant and with
the spatially non-uniform concentration of the chemo-attractant and with 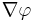 its gradient. Then the chemotactic cellular flow (also called current)
its gradient. Then the chemotactic cellular flow (also called current) 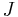 that is generated by the chemotaxis is linked to the above gradient by the law:
that is generated by the chemotaxis is linked to the above gradient by the law: 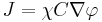 , where
, where 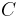 is the spatial density of the cells and
is the spatial density of the cells and 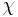 is the so-called ’Chemotactic coefficient’. However, note that in many cases
is the so-called ’Chemotactic coefficient’. However, note that in many cases 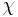 is not constant: it is, instead, a decreasing function of the concentration of the chemo-attractant
is not constant: it is, instead, a decreasing function of the concentration of the chemo-attractant 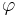 :
: 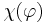 .
.
In the mirror of publications
Research of cell migration–as it was shown in the chapter ’History of chemotaxis research’–requires complementary application of classic and modern techniques. The field provides the possibility to present novel and valuable data in the basic research as well as in applied sciences. In the last 20–25 years, due to the factors mentioned above, there was an increase in number of publications dealing with itself the phenomenon chemotaxis. Nevertheless, other publications written in genetics, biochemistry, cell-physiology, pathology and clinical sciences could also incorporate data about migration or especially the chemotaxis of cells. A curiosity of migration research is that among several works investigating taxes (e.g.thermotaxis, geotaxis, phototaxis) chemotaxis research shows a significantly high ratio, which point to the underlined importance of chemotaxis research both in biology and medicine.
Measurement of chemotaxis
A wide range of techniques is available to evaluate chemotactic activity of cells or the chemoattractant and chemorepellent character of ligands. The basic requirements of the measurement are as follows:
- concentration gradients can develop relatively fast and persist for a long time in the system
- chemotactic and chemokinetic activities are distinguished
- migration of cells is free towards and away on the axis of the concentration gradient
- detected responses are the results of active migration of cells
Despite the fact that an ideal chemotaxis assay is still not available, there are several protocols and pieces of equipment which offer good correspondence with the conditions described above. The most commonly used are summarised in the table below:
| Type of assay | Agar-plate assays | Two-chamber assays | Others |
|---|---|---|---|
| Examples |
|
|
|
See also
References
- ^ Julius Adler and Wung-Wai Tso (1974). "Decision-Making in Bacteria: Chemotactic Response of Escherichia Coli to Conflicting Stimuli". Science 184 (4143): 1292–4. doi:10.1126/science.184.4143.1292. PMID 4598187.
- ^ U.K. Professor Captures Inaugural European Science Award, By Rob Knies, News - Microsoft Research, retrieved November 6, 2006Archive copy at the Wayback Machine
- ^ Computer bug study wins top prize, 3 November 2006, BBC NEWS, retrieved November 6, 2006
- ^ Howard C. Berg (2003). "E. coli in motion". Springer-Verlag, NY. (New York: Springer) ISBN 0-387-00888-8. ISBN 0387008888.
- ^ Bernardo A. Mello and Yuhai Tu (2007). "Effects of Adaptation in Maintaining High Sensitivity over a Wide Range of Backgrounds for Escherichia coli Chemotaxis". Biophysical Journal 92 (7): 2329–2337. doi:10.1529/biophysj.106.097808. PMC 1864821. PMID 17208965. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1864821.
- ^ Philippe Cluzel, Michael Surette and Stanislas Leibler (2000). "An Ultrasensitive Bacterial Motor Revealed by Monitoring Signaling Proteins in Single Cells". Science 287 (5458): 1652–1655. doi:10.1126/science.287.5458.1652. PMID 10698740.
- ^ Victor Sourjik; Tso, WW (2004). "Receptor clustering and signal processing in E. coli chemotaxis". TRENDS in Microbiology 12 (12): 569–576. doi:10.1016/j.tim.2004.10.003. PMID 15539117.
- ^ Anna Bagorda, Carole A. Parent (2008). "Eukaryotic chemotaxis at a glance". J. Cell Science 121 (Pt 16): 2621–4. doi:10.1242/jcs.018077. PMID 18685153.
- ^ Laszlo Kohidai (1999). "Chemotaxis: the proper physiological response to evaluate phylogeny of signal molecules". Acta Biol Hung 50 (4): 375–94. PMID 10735174.
- ^ Laszlo Kohidai and Gyorgy Csaba (1988). "Chemotaxis and chemotactic selection induced with cytokines (IL-8, RANTES and TNF alpha) in the unicellular Tetrahymena pyriformis.". Cytokine 10 (7): 481–6. doi:10.1006/cyto.1997.0328. PMID 9702410.
External links
- Chemotaxis (a book)
- Chemotaxis
- Neutrophil Chemotaxis
- Cell Migration Gateway
- Global Existence for Chemotaxis with Finite Sampling Radius
- Bacterial Chemotaxis Depends on a Two-Component Signaling Pathway Activated by Histidine-Kinase-associated Receptors, Molecular Biology of the Cell 4th Edition © 2002 by Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter.
- Figure 15-69. The two-component signaling pathway that enables chemotaxis receptors to control the flagellar motor during bacterial chemotaxis, Molecular Biology of the Cell 4th Edition © 2002 by Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter.
- Chemotaxis simulation downloadable from the Mathworks user-community, runs on Matlab
- Bacterial Chemotaxis Interactive Simulator - A web-app that features some simple algorithms for simulating bacterial chemotaxis.